| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-03-06 18:57:59 UTC |
|---|
| Update Date | 2014-12-24 20:20:58 UTC |
|---|
| Accession Number | T3D0048 |
|---|
| Identification |
|---|
| Common Name | Aroclor 1221 |
|---|
| Class | Small Molecule |
|---|
| Description | Aroclor 1221 is a commercial mixture of PCBs with an average chlorine content of 21%. It is composed of mainly monochlorobiphenyls (60.06%), and also includes bi-, tri, tetra-, and pentachlorinated homologs. Polychlorinated biphenyls (PCBs) are a group of 209 synthetic organic compounds with 1-10 chlorine atoms attached to biphenyl. PCBs were manufactured as commercial mixtures but banned in the 1970's because they were found to bioaccumulate in the environment and cause harmful health effects. However, PCBs do not break down readily and are still found in the environment. (9) |
|---|
| Compound Type | - Aromatic Hydrocarbon
- Coolant
- Industrial/Workplace Toxin
- Organic Compound
- Organochloride
- Plasticizer
- Pollutant
- Polychlorinated Biphenyl
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Arochlor 1221 | | PCB Aroclor 1221 | | PCB-1221 | | Polychorinated biphenyl 1221 |
|
|---|
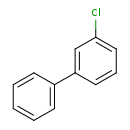
| Chemical Formula | C12H9Cl |
|---|
| Average Molecular Mass | 188.653 g/mol |
|---|
| Monoisotopic Mass | 188.039 g/mol |
|---|
| CAS Registry Number | 11104-28-2 |
|---|
| IUPAC Name | 1-chloro-3-phenylbenzene |
|---|
| Traditional Name | chlorobiphenyl |
|---|
| SMILES | ClC1=CC=CC(=C1)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C12H9Cl/c13-12-8-4-7-11(9-12)10-5-2-1-3-6-10/h1-9H |
|---|
| InChI Key | InChIKey=NMWSKOLWZZWHPL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as chlorinated biphenyls. These are organic compounds containing at least one chlorine atom attached to either benzene ring of the biphenyl moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Biphenyls and derivatives |
|---|
| Direct Parent | Chlorinated biphenyls |
|---|
| Alternative Parents | |
|---|
| Substituents | - Chlorinated biphenyl
- Halobenzene
- Chlorobenzene
- Aryl halide
- Aryl chloride
- Hydrocarbon derivative
- Organochloride
- Organohalogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Clear oil. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | 275–320 °C | | Solubility | 0.015 mg/mL at 25 °C [MABEY,WR et al. (1981)] | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-0900000000-3b0449d007885deaa670 | 2021-09-23 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0900000000-078debf47293a3078f91 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0900000000-52452caf77d9f8e3fb09 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0h2r-2900000000-f9982da6093a66e231db | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-2317f5aa7599807b806b | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0900000000-2317f5aa7599807b806b | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-0900000000-84060a760e32ca7b8c8c | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0900000000-11939ca1d475c7aef404 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0900000000-11939ca1d475c7aef404 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ug0-1900000000-4dfef4f0e7562aa54a5e | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-7e793e1a0ff1d221dc86 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0900000000-7e793e1a0ff1d221dc86 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f79-1900000000-57388ff8a5778a6bffb1 | 2021-10-12 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0f79-4900000000-54a8899979dcb8b378c7 | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100.41 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (9) ; inhalation (9) ; dermal (9) |
|---|
| Mechanism of Toxicity | The mechanism of action varies with the specific PCB. Dioxin-like PCBs bind to the aryl hydrocarbon receptor, which disrupts cell function by altering the transcription of genes, mainly be inducing the expression of hepatic Phase I and Phase II enzymes, especially of the cytochrome P450 family. Most of the toxic effects of PCBs are believed to be results of Ah receptor binding. Other PBCs are believed to interfere with calcium channels and/or change brain dopamine levels. PCBs can also cause endocrine disurption by altering the production of thyroid hormones and binding to estrogen receptors, which can stimulate the growth of certain cancer cells and produce other estrogenic effects, such as reproductive dysfunction. They will bioaccumulate by binding to receptor proteins such as uteroglobin. (1, 2, 5, 6) |
|---|
| Metabolism | PCBs are absorbed via inhalation, oral, and dermal routes of exposure. They are trasported in the blood, often bound to albumin. Due to their lipophilic nature they tend to accumulate in lipid-rich tissues, such as the liver, adipose, and skin. Metabolism of PCBs is very slow and varies based on the degree and position of chlorination. PCBs are metabolized by the microsomal monooxygenase system catalyzed by cytochrome P-450 enzymes to polar metabolites that can undergo conjugation with glutathione and glucuronic acid. The major metabolites are hydroxylated products which are excreted in the bile and faeces. The slow metabolism of PCBs means they tend to accumulate in body tissues. (9, 7) |
|---|
| Toxicity Values | LD50: 3980 mg/kg (Oral, Rat) (8) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 1, carcinogenic to humans. (11) |
|---|
| Uses/Sources | PCBs were used as coolants and lubricants in transformers, capacitors, and other electrical devices (such as fluorescent lights and refrigerators) produced before 1977. PCBs may contaminate the air and water near hazardouc waste sites. In addition, PCBs bioaccumulate in the environment and may be found in fish, meat, and dairy products. (9) |
|---|
| Minimum Risk Level | Intermediate Oral: 0.03 ug/kg/day (10) |
|---|
| Health Effects | The most common health effects of PCBs are skin conditions such as chloracne and rashes. Chronic PCB exposure has also been shown to cause liver, stomach and kidney, damage, jaundice, edema, anemia, changes in the immune system, behavioral alterations, and impaired reproduction. (9) |
|---|
| Symptoms | Chronic PCB exposure results in symptoms such as abdominal pain, nausea, vomiting, diarrhea, headache, dizziness, depression, nervousness, dermal and ocular lesions, fatigue, irregular menstrual cycles and a lowered immune response. (1) |
|---|
| Treatment | There are no specific treatments for PCB poisoning, since it is not usually recognized until after substantial chronic exposure. Only preventing further exposure and treating the observed symptoms can be done. Acute inhalation can be treated by administering oxygen. (9) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | Not Available |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | C032739 |
|---|
| Stitch ID | Aroclor 1221 |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D0048.pdf |
|---|
| General References | - Aoki Y: Polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans as endocrine disrupters--what we have learned from Yusho disease. Environ Res. 2001 May;86(1):2-11. [11386736 ]
- Safe S, Bandiera S, Sawyer T, Robertson L, Safe L, Parkinson A, Thomas PE, Ryan DE, Reik LM, Levin W, et al.: PCBs: structure-function relationships and mechanism of action. Environ Health Perspect. 1985 May;60:47-56. [2992927 ]
- Horowitz Y, Greenberg D, Ling G, Lifshitz M: Acrodynia: a case report of two siblings. Arch Dis Child. 2002 Jun;86(6):453. [12023189 ]
- Gill KD, Gupta V, Sandhir R: Ca2+/calmodulin-mediated neurotransmitter release and neurobehavioural deficits following lead exposure. Cell Biochem Funct. 2003 Dec;21(4):345-53. [14624473 ]
- Troisi GM, Haraguchi K, Kaydoo DS, Nyman M, Aguilar A, Borrell A, Siebert U, Mason CF: Bioaccumulation of polychlorinated biphenyls (PCBs) and dichlorodiphenylethane (DDE) methyl sulfones in tissues of seal and dolphin morbillivirus epizootic victims. J Toxicol Environ Health A. 2001 Jan 12;62(1):1-8. [11205532 ]
- Kester MH, Bulduk S, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Bergman A, Safe SH, Kuiper GG, Schuur AG, Brouwer A, Visser TJ: Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs. Endocrinology. 2000 May;141(5):1897-900. [10803601 ]
- World Health Organization (1993). Environ Health Criteria 140: Polychlorniated Biphenyls and Terphenyls.
- Lewis RJ (1996). Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold.
- ATSDR - Agency for Toxic Substances and Disease Registry (2000). Toxicological profile for polychlorinated biphenyls. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2001). Minimal Risk Levels (MRLs) for Hazardous Substances. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|