| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-03-06 18:58:02 UTC |
|---|
| Update Date | 2014-12-24 20:21:04 UTC |
|---|
| Accession Number | T3D0080 |
|---|
| Identification |
|---|
| Common Name | Dichloromethane |
|---|
| Class | Small Molecule |
|---|
| Description | Dichloromethane is used as an extraction solvent in the preparation of decaffeinated coffee, hop extracts and spice oleoresins. Diluent for colour additives and inks for marking fruit and vegetables The output of these processes is a mixture of methyl chloride, dichloromethane, chloroform, and carbon tetrachloride. These compounds are separated by distillation.
Dichloromethane has been shown to exhibit anti-tumor, anti-proliferative, analgesic, anti-fungal and antibiotic functions (1, 2, 3, 4, 5).
Dichloromethane belongs to the family of Organochlorides. These are organic compounds containing a chlorine atom. |
|---|
| Compound Type | - Food Toxin
- Household Toxin
- Industrial/Workplace Toxin
- Metabolite
- Organic Compound
- Organochloride
- Pesticide
- Pollutant
- Solvent
- Synthetic Compound
|
|---|
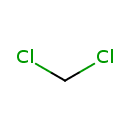
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Aerothene | | Aerothene MM | | CH2Cl2 | | DCM | | Dichloro-Methane | | Distillex DS3 | | Driverit | | Freon 30 | | M-Clean D | | Methane dichloride | | Methoklone | | Methylene bichloride | | Methylene chloride | | Methylene dichloride | | Narkotil | | Nevolin | | Salesthin | | Solaesthin | | Solmethine |
|
|---|
| Chemical Formula | CH2Cl2 |
|---|
| Average Molecular Mass | 84.933 g/mol |
|---|
| Monoisotopic Mass | 83.953 g/mol |
|---|
| CAS Registry Number | 1975-09-02 |
|---|
| IUPAC Name | dichloromethane |
|---|
| Traditional Name | methylene chloride |
|---|
| SMILES | ClCCl |
|---|
| InChI Identifier | InChI=1S/CH2Cl2/c2-1-3/h1H2 |
|---|
| InChI Key | InChIKey=YMWUJEATGCHHMB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as halomethanes. These are organic compounds in which at least one of the four hydrogen atoms of methane (CH4) are replaced by halogen atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organohalogen compounds |
|---|
| Class | Alkyl halides |
|---|
| Sub Class | Halomethanes |
|---|
| Direct Parent | Halomethanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Halomethane
- Hydrocarbon derivative
- Organochloride
- Alkyl chloride
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Colorless liquid. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | -96.8°C | | Boiling Point | Not Available | | Solubility | 13 mg/mL at 25°C | | LogP | 1.25 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0002-9000000000-fea8e21d8ce299fd6c73 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-000t-9000000000-3c727a0b79ead1d0afc9 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0002-9000000000-fea8e21d8ce299fd6c73 | 2018-05-18 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-000t-9000000000-3c727a0b79ead1d0afc9 | 2018-05-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-9000000000-a20182f85c9e395e5511 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-9000000000-9fdeb81f96b30affd700 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-9000000000-9fdeb81f96b30affd700 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9000000000-9fdeb81f96b30affd700 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-9000000000-3641e00693a6eab28168 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-9000000000-3641e00693a6eab28168 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9000000000-3641e00693a6eab28168 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-9000000000-cd27483dddc3a8ea1a54 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-9000000000-cd27483dddc3a8ea1a54 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9000000000-cd27483dddc3a8ea1a54 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-9000000000-fddbf58cbca8e803abce | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-9000000000-fddbf58cbca8e803abce | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001j-9000000000-ed36dd35150f69631b13 | 2021-09-22 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-000t-9000000000-6ce0b546ffcfe5b5cf7e | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 90 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (13) ; inhalation (13) ; dermal (13) |
|---|
| Mechanism of Toxicity | Methylene chloride targets the lungs, blood system, and nervous system. In the lungs its metabolites damage Clara cells. It is also metabolized into carbon monoxide, which binds to hemoglobin to produce dose-dependent increases in carboxyhemoglobin. This results in the reduced oxygen transport and neurological dysfunction characteristic of carboxyhemoglobinemia (carbon monoxide poisoning). Methylene chloride is also believed to cause neurotoxicity by interfering with signal transmission in a manner similar to general anesthetics. Certain metabolites, such as formaldehyde, may result in carcinogenic effects by causing DNA single strand breaks, DNA-protein crosslinks, and other mutations. (6, 13) |
|---|
| Metabolism | Absorption mainly occurs via inhalation, but may also result from oral or dermal exposure. Methylene chloride is mainly distributed to the adipose tissue and liver. It may be metabolized by cytochrome P-450 2E1, which ultimately produces carbon monoxide and carbon dioxide via formyl chloride. Methylene chloride can also be metabolized by theta glutathione-S-transferase, which produces carbon dioxide via a postulated glutathione conjugate (S-chloromethyl
glutathione) and formaldehyde. Both pathways produce toxic metabolites which are excreted mainly in expired air, but also in the urine. (6, 13) |
|---|
| Toxicity Values | LD50: 437 mg/kg (Intraperitoneal, Mouse) (7)
LD50: 6460 mg/kg (Subcutaneous, Mouse) (7)
LD50: 1600 mg/kg (Oral, Rat) (8)
LC50: 14 400 ppm over 7 hours (Inhalation, Mouse) (7) |
|---|
| Lethal Dose | 357 mg/kg (oral) or 50 000 ppm (inhalation) for an adult human. (9) |
|---|
| Carcinogenicity (IARC Classification) | 2A, probably carcinogenic to humans. (12) |
|---|
| Uses/Sources | Methylene chloride is widely used as a solvent in industrial processes, paint stripper, and degreaser. It is also used in food preparation, aerosol propellants, pesticides, and the manufacture of photographic film. (6, 13) |
|---|
| Minimum Risk Level | Acute Inhalation: 0.6 ppm (11)
Intermediate Inhalation: 0.3 ppm (11)
Chronic Inhalation: 0.3 ppm (11)
Acute Oral: 0.2 mg/kg/day (11)
Chronic Oral: 0.06 mg/kg/day (11) |
|---|
| Health Effects | Exposure to methylene chloride may cause optic neuropathy and hepatitis. Very high concentrations can lead to unconciousness, coma, and death. It is metabolized to carbon monoxide, potentially leading to carbon monoxide poisoning. Methylene chloride also causes liver and kidney injury, and may be a carcinogen. (6, 14) |
|---|
| Symptoms | Breathing large amounts of methylene chloride causes dizziness, nausea, tingling or numbness of the finger and toes, loss of concentration, and reduced hand-eye coordination. Very high concentrations can lead to unconciousness, coma, and death. Skin contact with methylene chloride causes burning and redness of the skin. (13, 14) |
|---|
| Treatment | Treatment of methylene chloride exposure is mainly symptomatic. Ingested methylene chloride may be removed by emesis and/or gastric lavage, and activated charcoal. Hyperbaric oxygen may be used to treat the carbon monoxide poisoning that can result from inhalation of methylene chloride. (15) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB31548 |
|---|
| PubChem Compound ID | 6344 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 6104 |
|---|
| KEGG ID | C02271 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 15767 |
|---|
| BioCyc ID | CPD-4521 |
|---|
| CTD ID | D008752 |
|---|
| Stitch ID | Methylene chloride |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 885 |
|---|
| Wikipedia Link | Dichloromethane |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D0080.pdf |
|---|
| General References | - Jin W, Shi Q, Hong C, Cheng Y, Ma Z, Qu H: Cytotoxic properties of thiophenes from Echinops grijissi Hance. Phytomedicine. 2008 Sep;15(9):768-74. Epub 2008 Feb 20. [18068965 ]
- Barreiro Arcos ML, Cremaschi G, Werner S, Coussio J, Ferraro G, Anesini C: Tilia cordata Mill. Extracts and scopoletin (isolated compound): differential cell growth effects on lymphocytes. Phytother Res. 2006 Jan;20(1):34-40. [16397918 ]
- Matheus ME, Berrondo LF, Vieitas EC, Menezes FS, Fernandes PD: Evaluation of the antinociceptive properties from Brillantaisia palisotii Lindau stems extracts. J Ethnopharmacol. 2005 Dec 1;102(3):377-81. Epub 2005 Aug 1. [16076537 ]
- Khan MR, Omoloso AD: Antibacterial and antifungal activities of Dracontomelon dao. Fitoterapia. 2002 Jul;73(4):327-30. [12234577 ]
- Meyer JJ, Dilika F: Antibacterial activity of Helichrysum pedunculatum used in circumcision rites. J Ethnopharmacol. 1996 Jul 26;53(1):51-4. [8807475 ]
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- Lewis RJ (1996). Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold.
- Verschueren K (1983). Handbook of Environmental Data of Organic Chemicals. 2nd ed. New York, NY: Van Nostrand Reinhold Co.
- Lewis RJ (2000). Sax's Dangerous Properties of Industrial Materials. 10th ed. New York, NY: Van Nostrand Reinhold Company.
- Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
- ATSDR - Agency for Toxic Substances and Disease Registry (2001). Minimal Risk Levels (MRLs) for Hazardous Substances. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2000). Toxicological profile for methylene chloride. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Dichloromethane. Last Updated 20 May 2009. [Link]
- International Programme on Chemical Safety (IPCS) INCHEM (1997). Poison Information Monograph for Methylene Chloride. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|