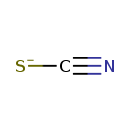| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-03-06 18:58:03 UTC |
|---|
| Update Date | 2014-12-24 20:21:05 UTC |
|---|
| Accession Number | T3D0089 |
|---|
| Identification |
|---|
| Common Name | Thiocyanate |
|---|
| Class | Small Molecule |
|---|
| Description | Thiocyanates are a group of compounds formed from a combination of sulfur, carbon, and nitrogen. Thiocyanates are found in various foods and plants and are produced primarily from the reaction of free cyanide with sulfur. This reaction occurs in the environment (for example, in industrial waste streams that contain cyanide) and in the human body after cyanide ingestion. Thiocyanates are present in water primarily because of discharges from coal processing, extraction of gold and silver, and mining industries. Thiocyanate is the major product formed from cyanide that passes into the body as the body attempts to rid itself of cyanide. (8) |
|---|
| Compound Type | - Cyanide Compound
- Food Toxin
- Industrial/Workplace Toxin
- Metabolite
- Natural Compound
- Nitrile
- Organic Compound
- Plant Toxin
- Pollutant
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Ammonium sulfocyanate | | Nitridosulfanidocarbon | | Nitrodithiocarbone(1-) | | Nitrodothiocarbonate | | Rhodanid | | Rhodanide | | SCN | | Thiocyanate ion | | Thiocyanic acid | | Thiocyanid | | Weedazol TL |
|
|---|
| Chemical Formula | CNS |
|---|
| Average Molecular Mass | 58.083 g/mol |
|---|
| Monoisotopic Mass | 57.976 g/mol |
|---|
| CAS Registry Number | 302-04-5 |
|---|
| IUPAC Name | cyanosulfanide |
|---|
| Traditional Name | thiocyanate |
|---|
| SMILES | [S-]C#N |
|---|
| InChI Identifier | InChI=1S/CHNS/c2-1-3/h3H/p-1 |
|---|
| InChI Key | InChIKey=ZMZDMBWJUHKJPS-UHFFFAOYSA-M |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thiocyanates. These are salts or esters of thiocyanic acid, with the general formula RSC#N (R=alkyl, aryl). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organosulfur compounds |
|---|
| Class | Thiocyanates |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Thiocyanates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Thiocyanate
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Organic anion
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Mitochondria
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Negative (Annotated) | splash10-0a4i-9000000000-afccae6819a2082a3b0e | 2012-07-24 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Negative (Annotated) | splash10-0a4i-9000000000-f7f42d61861dc8b47e0a | 2012-07-24 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Negative (Annotated) | splash10-0a4i-9000000000-d0c4694dff8128c82e83 | 2012-07-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-9000000000-96a5deef504fc8cb3052 | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9000000000-3714ea8b8485698022fe | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-a4aa9bc6ab8c5ca45d18 | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4l-9000000000-3e068a4808fd5db992f0 | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052f-9000000000-5b443ccd8993bc2f9325 | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9000000000-626420c56c66b85a0f64 | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-9000000000-286b63d3516de7d14a12 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9000000000-286b63d3516de7d14a12 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-286b63d3516de7d14a12 | 2021-09-22 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (8); inhalation (8) ; dermal (8) |
|---|
| Mechanism of Toxicity | Thiocyanate (sulphocyanate or SCN) is believed to be a goitrogenic compound. It is a competitive inhibitor of the human thyroid sodium/iodide symporter NIS. Thus, the adverse effects of thiocyanate overload are especially noticeable when iodine availability is low. Intake of goitrogenic substances causes an adaptive increase in T3‰ЫЄs binding to brain nuclear receptors and in the activity of type II 5'-deiodinase, which generates T3 from T4. This altered function and availability of T3 is detrimental to the developing brain. Thiocyanate is also known to modulate activity of mammalian peroxidases. For instance, eosinophil peroxidase has been implicated in promoting oxidative tissue damage in a variety of inflammatory conditions, including asthma. Thiocyanate also acts as inhibitor to carbonic anhydrase, which catalyzes the rapid conversion of carbon dioxide to bicarbonate and protons. (1, 4, 6) Organic nitriles decompose into cyanide ions both in vivo and in vitro. Consequently the primary mechanism of toxicity for organic nitriles is their production of toxic cyanide ions or hydrogen cyanide. Cyanide is an inhibitor of cytochrome c oxidase in the fourth complex of the electron transport chain (found in the membrane of the mitochondria of eukaryotic cells). It complexes with the ferric iron atom in this enzyme. The binding of cyanide to this cytochrome prevents transport of electrons from cytochrome c oxidase to oxygen. As a result, the electron transport chain is disrupted and the cell can no longer aerobically produce ATP for energy. Tissues that mainly depend on aerobic respiration, such as the central nervous system and the heart, are particularly affected. Cyanide is also known produce some of its toxic effects by binding to catalase, glutathione peroxidase, methemoglobin, hydroxocobalamin, phosphatase, tyrosinase, ascorbic acid oxidase, xanthine oxidase, succinic dehydrogenase, and Cu/Zn superoxide dismutase. Cyanide binds to the ferric ion of methemoglobin to form inactive cyanmethemoglobin. (14) |
|---|
| Metabolism | Thiocyanates can appear in the body after metabolization of cyanides by rhodanese. When thiocyanates enter the body, they normally breaks down in aqueous solution to yield sulfate ions. However, thiocyanates are also found in the thyroid fluids. Immediately following exposure to thiocyanate containing solutions, the cystic fibrosis conductance regulator Cl‰ЫТ channel exhibits high unitary SCN‰ЫТ conductance and anomalous mole fraction behaviour. Thiocyanates is normally excreted in urine. (2, 3, 4, 5) Organic nitriles are converted into cyanide ions through the action of cytochrome P450 enzymes in the liver. Cyanide is rapidly absorbed and distributed throughout the body. Cyanide is mainly metabolized into thiocyanate by either rhodanese or 3-mercaptopyruvate sulfur transferase. Cyanide metabolites are excreted in the urine. (13) |
|---|
| Toxicity Values | Guanidine Thiocyanate: LD50: 375 mg/kg (Oral, Rat), LD50: 2000 mg/kg (Dermal, Rabbit) (10)
Potassium Thiocyanate: LD50: 854 mg/kg (Oral, Rat) (11)
Ammonium Thiocyanate: LD50: 750 mg/kg (Oral, Rat), LD50: 500 mg/kg (Oral, Mouse) (12) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Exposure occurs from breathing air and drinking water, touching soil or water containing thiocyanate, or eating foods that contain thiocyanate. Thiocyanates are present in water primarily because of discharges from coal processing, extraction of gold and silver, and mining industries. Thiocyanate is the major product formed from cyanide that passes into the body as the body attempts to rid itself of cyanide, thus exposure to cyanide also results in exposure to thiocyanate. (8) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Thiocyanates are known to affect the thyroid glands, reducing the ability of the gland to produce hormones that are necessary for the normal function of the body. Exposure to high levels of cyanide for a short time harms the brain and heart and can even cause coma and death. (8) |
|---|
| Symptoms | Symptoms of thiocyanate exposure include rapid, deep breathing and shortness of breath, followed by convulsions (seizures) and loss of consciousness. (8) |
|---|
| Treatment | In cases of thiocyanate exposure, get fresh air and medical attention. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. If swallowed, do not induce vomiting but give large quantities of water. Immediately flush skin with plenty of water for at least 15 minutes in case of exposure to skin or the eyes. (9) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB01453 |
|---|
| PubChem Compound ID | 781 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 8961 |
|---|
| KEGG ID | C01755 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 18022 |
|---|
| BioCyc ID | HSCN |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Thiocyanate |
|---|
| PDB ID | SCN |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Thiocyanate |
|---|
| References |
|---|
| Synthesis Reference | Lang, Konrad. Thiocyanate formation in the animal body. II. Biochemische Zeitschrift (1933), 263 262-7. |
|---|
| MSDS | Link |
|---|
| General References | - van Dalen CJ, Kettle AJ: Substrates and products of eosinophil peroxidase. Biochem J. 2001 Aug 15;358(Pt 1):233-9. [11485572 ]
- Laurberg P, Nohr SB, Pedersen KM, Fuglsang E: Iodine nutrition in breast-fed infants is impaired by maternal smoking. J Clin Endocrinol Metab. 2004 Jan;89(1):181-7. [14715847 ]
- WOOD JL, WILLIAMS EF Jr: The metabolism of thiocyanate in the rat and its inhibition by propylthiouracil. J Biol Chem. 1949 Jan;177(1):59-67. [18107406 ]
- Taga I, Oumbe VA, Johns R, Zaidi MA, Yonkeu JN, Altosaar I: Youth of west-Cameroon are at high risk of developing IDD due to low dietary iodine and high dietary thiocyanate. Afr Health Sci. 2008 Sep;8(3):180-5. [19357747 ]
- Fragoso MA, Fernandez V, Forteza R, Randell SH, Salathe M, Conner GE: Transcellular thiocyanate transport by human airway epithelia. J Physiol. 2004 Nov 15;561(Pt 1):183-94. Epub 2004 Sep 2. [15345749 ]
- Innocenti A, Zimmerman S, Ferry JG, Scozzafava A, Supuran CT: Carbonic anhydrase inhibitors. Inhibition of the beta-class enzyme from the methanoarchaeon Methanobacterium thermoautotrophicum (Cab) with anions. Bioorg Med Chem Lett. 2004 Sep 6;14(17):4563-7. [15357993 ]
- Paul BD, Smith ML: Cyanide and thiocyanate in human saliva by gas chromatography-mass spectrometry. J Anal Toxicol. 2006 Oct;30(8):511-5. [17132244 ]
- ATSDR - Agency for Toxic Substances and Disease Registry (2000). Toxicological profile for thiocyanate. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Shanxi Friends Union Chemicals Co., Ltd. (2009). [Link]
- Promega (2006). Material Safety Data Sheet for Guanidine thiocynate. [Link]
- Flinn Scientific Inc. (2002).Material Safety Data Sheet for Potassium thiocyanate. [Link]
- ScienceLab.com (2009). Material Safety Data Sheet (MSDS) for Ammonium thiocyanate. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2006). Toxicological profile for cyanide. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|