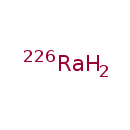| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-03-06 18:58:05 UTC |
|---|
| Update Date | 2014-12-24 20:21:06 UTC |
|---|
| Accession Number | T3D0100 |
|---|
| Identification |
|---|
| Common Name | Radium-226 |
|---|
| Class | Small Molecule |
|---|
| Description | Radium is a radioactive chemical element which has the symbol Ra and atomic number 88. Its appearance is almost pure white, but it readily oxidizes on exposure to air, turning black. Radium is an alkaline earth metal that is formed when uranium and thorium break down in the environment. It is extremely radioactive. Radium-226 is the most stable isotope, has a half-life of 1602 years, and decays into radon gas. Radium has been used as a radiation source for treating cancer, in radiography of metals, and combined with other metals as a neutron source for research and radiation instrument calibration. Until the 1960s, radium was a component of the luminous paints used for watch and clock dials, instrument panels in airplanes, military instruments, and compasses. (2, 3) |
|---|
| Compound Type | - Industrial/Workplace Toxin
- Inorganic Compound
- Metal
- Natural Compound
- Pollutant
- Radioactive
- Radioactive Isotope
- Radium Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 226Ra | | 88Ra | | Radium | | Radium 226 | | Radium, isotope of mass 226 |
|
|---|
| Chemical Formula | Ra |
|---|
| Average Molecular Mass | 226.025 g/mol |
|---|
| Monoisotopic Mass | 226.025 g/mol |
|---|
| CAS Registry Number | 13982-63-3 |
|---|
| IUPAC Name | (²²⁶Ra)radium |
|---|
| Traditional Name | (²²⁶Ra)radium |
|---|
| SMILES | [226Ra] |
|---|
| InChI Identifier | InChI=1S/Ra/i1+0 |
|---|
| InChI Key | InChIKey=HCWPIIXVSYCSAN-IGMARMGPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Classification | Not classified |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | Its appearance is almost pure white, but it readily oxidizes on exposure to air, turning black. (2) |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 973°K (700°C , 1292°F ) | | Boiling Point | 1737 °C | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | Not Available |
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Inhalation (2) ; injection (2) ; oral (2) ; dermal (2) |
|---|
| Mechanism of Toxicity | Ionizing radiation produced by radium damages the DNA, resulting in gene mutations and chromosomal aberrations. This can both initiate and promote carcinogenesis, and interfere with reproduction and development. Since radium`s similarity to calcium allows it to deposit in the bones, bone cancer is of particular risk. (3) |
|---|
| Metabolism | Due to its radioactivity, radium can affect the body following ingestion, inhalation, or dermal exposure. If inhalated, it may accumulate in the lungs. Once in the body radium may deposit in the bones, mainly on the surface and areas where new bone is being formed. Radium is not metabolized and is excreted primarily in the faeces. (3) |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 1, carcinogenic to humans. (1) |
|---|
| Uses/Sources | Radium has been used as a radiation source for treating cancer, in radiography of metals, and combined with other metals as a neutron source for research and radiation instrument calibration. Until the 1960s, radium was a component of the luminous paints used for watch and clock dials, instrument panels in airplanes, military instruments, and compasses. (3) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Radium is highly radioactive and its decay product, radon gas, is also radioactive. It has been shown to cause effects on the blood (anemia) and eyes (cataracts). Inhalation, injection, ingestion or body exposure to radium can cause cancer and other disorders, due to its radioactivity. Since radium is chemically similar to calcium, it has the potential to cause great harm by replacing it in bones, and bone cancer is of particular risk. (2, 3) |
|---|
| Symptoms | Exposure to high doses of ionizing radiation results in acute radiation syndrome, which can cause skin burns, hair loss, nausea, vomiting, dizziness, disorientation, low blood pressure, headache, fatigue, weakness, fever, birth defects, illness, infection, and death. (4, 5) |
|---|
| Treatment | Treatment reversing the effects of irradiation is currently not possible. Anaesthetics and antiemetics are administered to counter the symptoms of exposure, as well as antibiotics for countering secondary infections due to the resulting immune system deficiency. (5) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 6328144 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | Not Available |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 33325 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | D011883 |
|---|
| Stitch ID | Radium-226 |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 6526 |
|---|
| Wikipedia Link | Radium |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D0100.pdf |
|---|
| General References | - International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
- Wikipedia. Radium. Last Updated 24 June 2009. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (1990). Toxicological profile for radium. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (1999). Toxicological profile for ionizing radiation. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Radiation poisoning. Last Updated 22 August 2009. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|