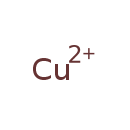| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-03-06 18:58:08 UTC |
|---|
| Update Date | 2014-12-24 20:21:10 UTC |
|---|
| Accession Number | T3D0128 |
|---|
| Identification |
|---|
| Common Name | Copper |
|---|
| Class | Small Molecule |
|---|
| Description | Copper is an essential nutrient to all higher plants and animals. Physiologically, it exists as an ion in the body. In animals, it is found primarily in the bloodstream, as a cofactor in various enzymes, and in copper-based pigments. In the body, copper shifts between the cuprous (Cu1+) and cupric (Cu2+) forms, though the majority of the body's copper is in the Cu2+ form. The ability of copper to easily accept and donate electrons explains its important role in oxidation-reduction (redox) reactions and in scavenging free radicals. Copper is a critical functional component of a number of essential enzymes known as cuproenzymes. For instance, the copper-dependent enzyme, cytochrome c oxidase, plays a critical role in cellular energy production. By catalyzing the reduction of molecular oxygen (O2) to water (H2O), cytochrome c oxidase generates an electrical gradient used by the mitochondria to create the vital energy-storing molecule, ATP. Another cuproenzyme, lysyl oxidase, is required for the cross-linking of collagen and elastin, which are essential for the formation of strong and flexible connective tissue. Another cuproeznyme, Monoamine oxidase (MAO), plays a role in the metabolism of the neurotransmitters norepinephrine, epinephrine, and dopamine. MAO also functions in the degradation of the neurotransmitter serotonin, which is the basis for the use of MAO inhibitors as antidepressants. One of the most important cuproenzymes is Superoxide dismutase (SOD). SOD functions as an antioxidant by catalyzing the conversion of superoxide radicals (free radicals or ROS) to hydrogen peroxide, which can subsequently be reduced to water by other antioxidant enzymes. Two forms of SOD contain copper: 1) copper/zinc SOD is found within most cells of the body, including red blood cells, and 2) extracellular SOD is a copper-containing enzyme found at high levels in the lungs and low levels in blood plasma. In sufficient amounts, copper can be poisonous or even fatal to organisms. Copper is normally bound to cuproenzymes (such as SOD, MOA) and is thus only toxic when unsequestered and unmediated. It is believed that zinc and copper compete for absorption in the digestive tract so that a diet that is excessive in one of these minerals may result in a deficiency in the other. An imbalance of zinc and copper status might be involved in human hypertension. |
|---|
| Compound Type | - Copper Compound
- Food Toxin
- Household Toxin
- Industrial/Workplace Toxin
- Inorganic Compound
- Metabolite
- Metal
- Natural Compound
- Pollutant
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Copper ion | | Copper(2+) | | Copper(2+) ion | | Copper(II) | | Copper(II) cation | | Copper(II) ion | | Cu(2+) | | Cu2+ |
|
|---|
| Chemical Formula | Cu |
|---|
| Average Molecular Mass | 63.545 g/mol |
|---|
| Monoisotopic Mass | 62.929 g/mol |
|---|
| CAS Registry Number | 7440-50-8 |
|---|
| IUPAC Name | copper(2+) ion |
|---|
| Traditional Name | copper(2+) ion |
|---|
| SMILES | [Cu++] |
|---|
| InChI Identifier | InChI=1S/Cu/q+2 |
|---|
| InChI Key | InChIKey=JPVYNHNXODAKFH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as homogeneous transition metal compounds. These are inorganic compounds containing only metal atoms,with the largest atom being a transition metal atom. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Homogeneous metal compounds |
|---|
| Class | Homogeneous transition metal compounds |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Homogeneous transition metal compounds |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | - Homogeneous transition metal
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | - Brain
- Erythrocyte
- Hair
- Intestine
- Kidney
- Liver
|
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | Reddish metallic solid. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 1083°C | | Boiling Point | 2595°C (4703°F) | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-9000000000-59c652eccc13cc365f65 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-9000000000-59c652eccc13cc365f65 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-9000000000-59c652eccc13cc365f65 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-9000000000-9acd78ab9faeb89677a7 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-9000000000-9acd78ab9faeb89677a7 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-9000000000-9acd78ab9faeb89677a7 | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (27) ; inhalation (27) ; dermal (27) |
|---|
| Mechanism of Toxicity | Excess copper is sequestered within hepatocyte lysosomes, where it is complexed with metallothionein. Copper hepatotoxicity is believed to occur when the lysosomes become saturated and copper accumulates in the nucleus, causing nuclear damage. This damage is possibly a result of oxidative damage, including lipid peroxidation. Copper inhibits the sulfhydryl group enzymes such as glucose-6-phosphate 1-dehydrogenase, glutathione reductase, and paraoxonases, which protect the cell from free oxygen radicals. It also influences gene expression and is a co-factor for oxidative enzymes such as cytochrome C oxidase and lysyl oxidase. In addition, the oxidative stress induced by copper is thought to activate acid sphingomyelinase, which lead to the production of ceramide, an apoptotic signal, as well as cause hemolytic anemia. Copper-induced emesis results from stimulation of the vagus nerve. (27, 25, 1, 30) |
|---|
| Metabolism | Copper is mainly absorbed through the gastrointestinal tract, but it can also be inhalated and absorbed dermally. It passes through the basolateral membrane, possibly via regulatory copper transporters, and is transported to the liver and kidney bound to serum albumin. The liver is the critical organ for copper homeostasis. In the liver and other tissues, copper is stored bound to metallothionein, amino acids, and in association with copper-dependent enzymes, then partitioned for excretion through the bile or incorporation into intra- and extracellular proteins. The transport of copper to the peripheral tissues is accomplished through the plasma attached to serum albumin, ceruloplasmin or low-molecular-weight complexes. Copper may induce the production of metallothionein and ceruloplasmin. The membrane-bound copper transporting adenosine triphosphatase (Cu-ATPase) transports copper ions into and out of cells. Physiologically normal levels of copper in the body are held constant by alterations in the rate and amount of copper absorption, compartmental distribution, and excretion. (27, 29) |
|---|
| Toxicity Values | LD50: 3500 ug/kg (Intraperitoneal, Mouse) (24) |
|---|
| Lethal Dose | 10 to 20 grams for an adult human. (23) |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Copper is used as a thermal conductor, an electrical conductor, a building material, and a constituent of various metal alloys such as brass and bronze. Copper compounds have been widely used historically as pigments in decorative art. Copper compounds are also commonly used in agriculture to treat plant diseases like mildew, for water treatment, and as preservatives for wood, leather, and fabrics. (27, 28) |
|---|
| Minimum Risk Level | Acute Oral: 0.01 mg/kg/day (26)
Intermediate Oral: 0.01 mg/kg/day (26) |
|---|
| Health Effects | Copper toxicity, also called copperiedus, refers to the consequences of an excess of copper in the body. Copperiedus can occur from eating acid foods cooked in uncoated copper cookware, or from exposure to excess copper in drinking water or other environmental sources. Very-high doses of copper can damage liver and kidneys, and can even cause death. Copper may induce allergic responses in sensitive individuals. (28, 29) |
|---|
| Symptoms | Breathing high levels of copper can cause irritation of the nose and throat. Acute symptoms of copper poisoning by ingestion include vomiting, hematemesis (vomiting of blood), hypotension (low blood pressure), melena (black "tarry" feces), coma, jaundice (yellowish pigmentation of the skin), and gastrointestinal distress. Individuals with glucose-6-phosphate deficiency may be at increased risk of hematologic effects of copper. Hemolytic anemia resulting from the treatment of burns with copper compounds is infrequent. Chronic (long-term) effects of copper exposure can damage the liver and kidneys. |
|---|
| Treatment | In cases of suspected copper poisoning, penicillamine is the drug of choice, and dimercaprol, a heavy metal chelating agent, is often administered. Vinegar is not recommended, as it assists in solubilizing insoluble copper salts. |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB00657 |
|---|
| PubChem Compound ID | 23978 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 25221 |
|---|
| KEGG ID | C00070 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | 121270147450215600309400602268603085603088603735603864607238610101 |
|---|
| ChEBI ID | 30052 |
|---|
| BioCyc ID | CUCL2 |
|---|
| CTD ID | D003300 |
|---|
| Stitch ID | Copper |
|---|
| PDB ID | CU |
|---|
| ACToR ID | 6411 |
|---|
| Wikipedia Link | Copper |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Link |
|---|
| General References | - Brewer GJ: A brand new mechanism for copper toxicity. J Hepatol. 2007 Oct;47(4):621-2. Epub 2007 Jul 23. [17697726 ]
- Bardsley PA, Howard P, DeBacker W, Vermeire P, Mairesse M, Ledent C, Radermecker M, Bury T, Ansquer J: Two years treatment with almitrine bismesylate in patients with hypoxic chronic obstructive airways disease. Eur Respir J. 1991 Mar;4(3):308-10. [1907566 ]
- Koury JC, de Olilveria AV Jr, Portella ES, de Olilveria CF, Lopes GC, Donangelo CM: Zinc and copper biochemical indices of antioxidant status in elite athletes of different modalities. Int J Sport Nutr Exerc Metab. 2004 Jun;14(3):358-72. [15256695 ]
- Hoogenraad TU: Paradigm shift in treatment of Wilson's disease: zinc therapy now treatment of choice. Brain Dev. 2006 Apr;28(3):141-6. Epub 2006 Feb 7. [16466879 ]
- Kedzierska E: [Concentrations of selected bioelements and toxic metals and their influence on health status of children and youth residing in Szczecin]. Ann Acad Med Stetin. 2003;49:131-43. [15552844 ]
- Dib N, Valsesia E, Malinge MC, Mauras Y, Misrahi M, Cales P: Late onset of Wilson's disease in a family with genetic haemochromatosis. Eur J Gastroenterol Hepatol. 2006 Jan;18(1):43-7. [16357618 ]
- Kodama H, Sato E, Gu YH, Shiga K, Fujisawa C, Kozuma T: Effect of copper and diethyldithiocarbamate combination therapy on the macular mouse, an animal model of Menkes disease. J Inherit Metab Dis. 2005;28(6):971-8. [16435190 ]
- Cengiz B, Soylemez F, Ozturk E, Cavdar AO: Serum zinc, selenium, copper, and lead levels in women with second-trimester induced abortion resulting from neural tube defects: a preliminary study. Biol Trace Elem Res. 2004 Mar;97(3):225-35. [14997023 ]
- Langner C, Denk H: Wilson disease. Virchows Arch. 2004 Aug;445(2):111-8. Epub 2004 Jun 17. [15205951 ]
- Kitzberger R, Madl C, Ferenci P: Wilson disease. Metab Brain Dis. 2005 Dec;20(4):295-302. [16382340 ]
- Chen D, Cui QC, Yang H, Dou QP: Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006 Nov 1;66(21):10425-33. [17079463 ]
- Briviba K, Schnabele K, Rechkemmer G, Bub A: Supplementation of a diet low in carotenoids with tomato or carrot juice does not affect lipid peroxidation in plasma and feces of healthy men. J Nutr. 2004 May;134(5):1081-3. [15113949 ]
- Pizent A, Jurasovic J, Telisman S: Serum calcium, zinc, and copper in relation to biomarkers of lead and cadmium in men. J Trace Elem Med Biol. 2003;17(3):199-205. [14968933 ]
- Squitti R, Barbati G, Rossi L, Ventriglia M, Dal Forno G, Cesaretti S, Moffa F, Caridi I, Cassetta E, Pasqualetti P, Calabrese L, Lupoi D, Rossini PM: Excess of nonceruloplasmin serum copper in AD correlates with MMSE, CSF [beta]-amyloid, and h-tau. Neurology. 2006 Jul 11;67(1):76-82. [16832081 ]
- Odland JO, Nieboer E, Romanova N, Thomassen Y: Elements in placenta and pregnancy outcome in arctic and subarctic areas. Int J Circumpolar Health. 2004 May;63(2):169-87. [15253483 ]
- Venelinov TI, Davies IM, Beattie JH: Dialysis-Chelex method for determination of exchangeable copper in human plasma. Anal Bioanal Chem. 2004 Jul;379(5-6):777-80. Epub 2004 Feb 26. [14991216 ]
- Attri S, Sharma N, Jahagirdar S, Thapa BR, Prasad R: Erythrocyte metabolism and antioxidant status of patients with Wilson disease with hemolytic anemia. Pediatr Res. 2006 Apr;59(4 Pt 1):593-7. [16549536 ]
- Jablonska-Kaszewska I, Dabrowska E, Drobinska Jurowiecka A, Falkiewicz B: Treatment of Wilson's disease. Med Sci Monit. 2003 Aug;9 Suppl 3:5-8. [15156602 ]
- Daniel KG, Harbach RH, Guida WC, Dou QP: Copper storage diseases: Menkes, Wilsons, and cancer. Front Biosci. 2004 Sep 1;9:2652-62. [15358588 ]
- Aoki T: [Genetic disorders of copper transport--diagnosis and new treatment for the patients of Wilson's disease]. No To Hattatsu. 2005 Mar;37(2):99-109. [15773321 ]
- Meng Y, Miyoshi I, Hirabayashi M, Su M, Mototani Y, Okamura T, Terada K, Ueda M, Enomoto K, Sugiyama T, Kasai N: Restoration of copper metabolism and rescue of hepatic abnormalities in LEC rats, an animal model of Wilson disease, by expression of human ATP7B gene. Biochim Biophys Acta. 2004 Nov 5;1690(3):208-19. [15511628 ]
- Gorter RW, Butorac M, Cobian EP: Examination of the cutaneous absorption of copper after the use of copper-containing ointments. Am J Ther. 2004 Nov-Dec;11(6):453-8. [15543084 ]
- Baselt RC (2000). Disposition of Toxic Drugs and Chemicals in Man, 5th ed. Foster City, CA: Chemical Toxicology Institute.
- National Institute for Occupational Safety and Health (2002). RTECS: Registry of Toxic Effects of Chemical Substances.
- Baxter PJ, Adams PH, & Aw TC (2000). Hunter's Diseases of Occupations. 9th ed. New York, NY: Oxford University Press Inc.
- ATSDR - Agency for Toxic Substances and Disease Registry (2001). Minimal Risk Levels (MRLs) for Hazardous Substances. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Copper. Last Updated 29 May 2009. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2004). Toxicological profile for copper. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- International Programme on Chemical Safety (IPCS) INCHEM (1998). Environmental Health Criteria for Copper. [Link]
- US Environmental Protection Agency (2008). Drinking Water Health Advisory for 2,4-Dinitrotoluene and 2,6-Dinitrotoluene. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|