| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-03-06 18:58:11 UTC |
|---|
| Update Date | 2014-12-24 20:21:14 UTC |
|---|
| Accession Number | T3D0156 |
|---|
| Identification |
|---|
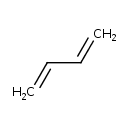
| Common Name | 1,3-Butadiene |
|---|
| Class | Small Molecule |
|---|
| Description | 1,3-Butadiene is a simple conjugated diene with the formula C4H6. It is an important industrial chemical used as a monomer in the production of synthetic rubber. When the word butadiene is used, most of the time it refers to 1,3-butadiene. 1,3-Butadiene is inconvenient for laboratory use because it is a flammable gas subject to polymerization on storage. 3-Butadiene cyclic sulfone (sulfolene) is a convenient solid storable source for 1,3-butadiene for many laboratory purposes when the generation of sulfur dioxide byproduct in the reaction mixture is not objectionable. Long-term exposure has been associated with cardiovascular disease, there is a consistent association with leukemia, and weaker association with other cancers. Most butadiene is polymerized to produce synthetic rubber. While polybutadiene itself is a very soft, almost liquid material, copolymers prepared from mixtures of butadiene with styrene and/or acrylonitrile, such as acrylonitrile butadiene styrene (ABS), acrylonitrile butadiene (NBR) and styrene-butadiene (SBR) are tough and elastic. SBR is the material most commonly used for the production of automobile tires. Smaller amounts of butadiene are used to make the nylon intermediate, adiponitrile, by the addition of a molecule of hydrogen cyanide to each of the double bonds in a process called hydrocyanation developed by DuPont. Other synthetic rubber materials such as chloroprene, and the solvent sulfolane are also manufactured from butadiene. Butadiene is used in the industrial production of 4-vinylcyclohexene via a Diels Alder dimerization reaction and the vinylcyclohexene is a common impurity found in butadiene upon storage. Cyclooctadiene and cyclododecatriene are produced via nickel- or titanium-catalyzed dimerization and trimerization reactions, respectively. Butadiene is also useful in the synthesis of cycloalkanes and cycloalkenes, as it reacts with double and triple carbon-carbon bonds through the Diels-Alder reaction. The name butadiene can also refer to the isomer, 1,2-butadiene, which is a cumulated diene. However, this allene is difficult to prepare and has no industrial significance. This diene is also not expected to act as a diene in a Diels-Alder reaction due to its structure. To effect a Diels-Alder reaction only a conjugated diene will suffice. |
|---|
| Compound Type | - Cigarette Toxin
- Household Toxin
- Industrial Precursor/Intermediate
- Industrial/Workplace Toxin
- Lachrymator
- Metabolite
- Organic Compound
- Pollutant
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1,3-Butadien | | alpha,gamma-Butadiene | | alpha,«gamma»-Butadiene | | Biethylene | | Bivinyl | | Buta-1,3-dieen | | Buta-1,3-dien | | Buta-1,3-diene | | Butadieen | | Butadien | | Butadiene | | CH22CH1CH2CH2 | | CH2=CHCH=CH2 | | Divinyl | | Erythrene | | Pyrrolylene | | Vinylethylene |
|
|---|
| Chemical Formula | C4H6 |
|---|
| Average Molecular Mass | 54.090 g/mol |
|---|
| Monoisotopic Mass | 54.047 g/mol |
|---|
| CAS Registry Number | 106-99-0 |
|---|
| IUPAC Name | buta-1,3-diene |
|---|
| Traditional Name | 1,3-butadiene |
|---|
| SMILES | C=CC=C |
|---|
| InChI Identifier | InChI=1S/C4H6/c1-3-4-2/h3-4H,1-2H2 |
|---|
| InChI Key | InChIKey=KAKZBPTYRLMSJV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alkadienes. These are acyclic hydrocarbons that contain exactly two carbon-to-carbon double bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Hydrocarbons |
|---|
| Class | Unsaturated hydrocarbons |
|---|
| Sub Class | Olefins |
|---|
| Direct Parent | Alkadienes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkadiene
- Unsaturated aliphatic hydrocarbon
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Colorless gas. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | -108.9°C | | Boiling Point | Not Available | | Solubility | 0.735 mg/mL at 25°C | | LogP | 1.99 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0ufr-9000000000-cddbfceffd529008d5ec | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0ufr-9000000000-cddbfceffd529008d5ec | 2018-05-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-9000000000-e4f51ef2fbf933007d39 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-9000000000-805abc61b7c8de6c5789 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9000000000-805abc61b7c8de6c5789 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-46aa55de9e12fa462b49 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-9000000000-191296d0bc49a4a79c02 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-9000000000-191296d0bc49a4a79c02 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-9000000000-7031b766ca514dc531b4 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-9000000000-0d737e29499be7940207 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-9000000000-0d737e29499be7940207 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-9000000000-0d737e29499be7940207 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-9000000000-1924391f943e092b4486 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9000000000-c60974066ebc2547dd57 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f79-9000000000-45337fddf4aab37b4ba0 | 2021-09-22 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0udi-9000000000-cfc5558b93570d1d78c0 | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, CCl4, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-24 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Inhalation (8) |
|---|
| Mechanism of Toxicity | Certain metabolites of 1,3-butadiene have been shown to bind to DNA and nucleoproteins, forming protein-DNA and DNA-DNA crosslinks. Specifically, 1,2-epoxybutene-3 and diepoxybutane react with guanine to cause crosslinking. (8, 1) |
|---|
| Metabolism | 1,3-Butadiene is absorbed following inhalation and is distributed to the adipose tissue, brain, liver, septum, and kidney. 1,3-Butadiene is believed to be metabolized in the liver by cytochrome P-450 enzymes, forming 1,2-epoxybutene-3 as the main metabolite. 1,2-Epoxybutene-3 is further transformed into 3-butene-1,2-diol by microsomal epoxide hydrolase. The metabolites of 1,3-butadiene are exhaled as carbon dioxide or excreted in the urine. (8) |
|---|
| Toxicity Values | LD50: 3.21 g/kg (Oral, Mouse) (4)
LC50: 270 000 mg/m3 over 2 hours (Inhalation, Mouse) (4) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 1, carcinogenic to humans. (7) |
|---|
| Uses/Sources | 1,3-Butadiene is made from the processing of petroleum. It is used mainly to make synthetic rubber for tires. It is also used to make plastics such as acrylics, and small amounts can be found in gasoline. (8) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Breathing high levels of 1,3-butadiene causes central nervous system damage. Chronic exposure may also cause lung damage and kidney, liver, and cardiovascular disease. In addition, 1,3-butadiene is a known human carcinogen. (8) |
|---|
| Symptoms | Breathing 1,3-butadiene may cause irritation of the eyes, nose, and throat. High levels of 1,3-butadiene can also cause, blurred vision, nausea, fatigue, headache, decreased blood pressure and pulse rate, and unconsciousness. Skin contact with liquid 1,3-butadiene can cause irritation and frostbite. (8) |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB41792 |
|---|
| PubChem Compound ID | 7845 |
|---|
| ChEMBL ID | CHEMBL537970 |
|---|
| ChemSpider ID | 7557 |
|---|
| KEGG ID | C16450 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 39478 |
|---|
| BioCyc ID | BUTADIENE |
|---|
| CTD ID | C031763 |
|---|
| Stitch ID | 1,3-Butadiene |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 204 |
|---|
| Wikipedia Link | 1,3-butadiene |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - Goggin M, Loeber R, Park S, Walker V, Wickliffe J, Tretyakova N: HPLC-ESI+-MS/MS analysis of N7-guanine-N7-guanine DNA cross-links in tissues of mice exposed to 1,3-butadiene. Chem Res Toxicol. 2007 May;20(5):839-47. Epub 2007 Apr 25. [17455958 ]
- Boysen G, Scarlett CO, Temple B, Combs TP, Brooks NL, Borchers CH, Swenberg JA: Identification of covalent modifications in P450 2E1 by 1,2-epoxy-3-butene in vitro. Chem Biol Interact. 2007 Mar 20;166(1-3):170-5. Epub 2007 Jan 17. [17298833 ]
- Norppa H: Genetic susceptibility, biomarker respones, and cancer. Mutat Res. 2003 Nov;544(2-3):339-48. [14644336 ]
- Ollagnier-de Choudens S, Fontecave M: The lipoate synthase from Escherichia coli is an iron-sulfur protein. FEBS Lett. 1999 Jun 18;453(1-2):25-8. [10403368 ]
- Zhang ZC, Zhang X, Yu QY, Liu ZC, Xu CM, Gao JS, Zhuang J, Wang X: Pd cluster nanowires as highly efficient catalysts for selective hydrogenation reactions. Chemistry. 2012 Feb 27;18(9):2639-45. doi: 10.1002/chem.201102903. Epub 2012 Jan 26. [22282407 ]
- USEPA (1985). Health Assessment Document: 1,3-Butadiene. EPA-600/8-85-004A.
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (1995). Toxicological Profile for 1,3-butadiene . U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|