| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-03-06 18:58:14 UTC |
|---|
| Update Date | 2014-12-24 20:21:18 UTC |
|---|
| Accession Number | T3D0185 |
|---|
| Identification |
|---|
| Common Name | p-Xylene |
|---|
| Class | Small Molecule |
|---|
| Description | p-Xylene is an aromatic hydrocarbon based on benzene with two methyl substituents with the chemical formula C8H10 or C6H4(CH3)2. The p stands for para, identifying the location of the methyl groups as across from one another. Overexposure of p-xylene in humans can cause headache, fatigue, dizziness, listlessness, confusion, irritability, gastrointestinal disturbances including nausea and loss of appetite, flushing of the face, and a feeling of increased body heat. p-Xylene vapor exposure over the recommended exposure limit of 100 parts per million (ppm) can cause irritation to eye, nose, and throat and possible chest tightening and an abnormal gait. |
|---|
| Compound Type | - Aromatic Hydrocarbon
- Food Toxin
- Household Toxin
- Industrial/Workplace Toxin
- Lachrymator
- Metabolite
- Natural Compound
- Organic Compound
- Pollutant
- Solvent
|
|---|
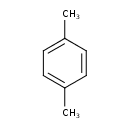
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1,4-Dimethylbenzene | | 1,4-Dimethylbenzol | | 1,4-xylene | | 4-methyltoluene | | p-dimethylbenzene | | p-Methyltoluene | | P-Xylene | | p-Xylol | | para-xylene |
|
|---|
| Chemical Formula | C8H10 |
|---|
| Average Molecular Mass | 106.165 g/mol |
|---|
| Monoisotopic Mass | 106.078 g/mol |
|---|
| CAS Registry Number | 106-42-3 |
|---|
| IUPAC Name | 1,4-xylene |
|---|
| Traditional Name | para-xylene |
|---|
| SMILES | CC1=CC=C(C)C=C1 |
|---|
| InChI Identifier | InChI=1S/C8H10/c1-7-3-5-8(2)6-4-7/h3-6H,1-2H3 |
|---|
| InChI Key | InChIKey=URLKBWYHVLBVBO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as p-xylenes. These are aromatic compounds that contain a p-xylene moiety, which is a monocyclic benzene carrying exactly two methyl groups at the 1- and 4-positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Xylenes |
|---|
| Direct Parent | p-Xylenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - P-xylene
- Aromatic hydrocarbon
- Unsaturated hydrocarbon
- Hydrocarbon
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Colorless liquid. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 13.2°C | | Boiling Point | Not Available | | Solubility | 0.162 mg/mL at 25 °C [SANEMASA,I et al. (1982)] | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-052f-9400000000-4b2097f841009fc7a1f9 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-052f-9400000000-58e5d57804187b27c6c5 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - CI-B (Non-derivatized) | splash10-0a4i-0900000000-3bf7450495bd2c1b9ae3 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-052f-9400000000-4b2097f841009fc7a1f9 | 2018-05-18 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-052f-9400000000-58e5d57804187b27c6c5 | 2018-05-18 | View Spectrum | | GC-MS | GC-MS Spectrum - CI-B (Non-derivatized) | splash10-0a4i-0900000000-3bf7450495bd2c1b9ae3 | 2018-05-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-4900000000-1ec9768719ddef2a0c60 | 2017-09-20 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0900000000-4772806c99a8f48026de | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0900000000-8925e888b29ad16f979b | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pdi-9300000000-cad6676e80b49deb4ca3 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0900000000-987fa9bb840efccd55d2 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0900000000-987fa9bb840efccd55d2 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-4900000000-5f8059855d9428f65159 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-4900000000-4ad4cb819bede18f8cf0 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-9100000000-cd6c0b30c7e9933348f5 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fvl-9000000000-a902105af7410297b68e | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0900000000-861947f0491f909a2588 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0900000000-861947f0491f909a2588 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-3900000000-e442490a7749a4a80a2a | 2021-10-12 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-052f-9500000000-313b5b919e2804de352e | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 25.16 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (12) ; inhalation (12) ; dermal (12) |
|---|
| Mechanism of Toxicity | p-Xylene is a cholinesterase or acetylcholinesterase (AChE) inhibitor. A cholinesterase inhibitor (or 'anticholinesterase') suppresses the action of acetylcholinesterase. Because of its essential function, chemicals that interfere with the action of acetylcholinesterase are potent neurotoxins, causing excessive salivation and eye-watering in low doses, followed by muscle spasms and ultimately death. Nerve gases and many substances used in insecticides have been shown to act by binding a serine in the active site of acetylcholine esterase, inhibiting the enzyme completely. Acetylcholine esterase breaks down the neurotransmitter acetylcholine, which is released at nerve and muscle junctions, in order to allow the muscle or organ to relax. The result of acetylcholine esterase inhibition is that acetylcholine builds up and continues to act so that any nerve impulses are continually transmitted and muscle contractions do not stop. Among the most common acetylcholinesterase inhibitors are phosphorus-based compounds, which are designed to bind to the active site of the enzyme. The structural requirements are a phosphorus atom bearing two lipophilic groups, a leaving group (such as a halide or thiocyanate), and a terminal oxygen. |
|---|
| Metabolism | Xylenes are well absorbed by the inhalation and oral routes, and to a much lesser extent by dermal route. Xylene is rapidly distributed throughout the body via the systemic circulation. Its metabolites are excreted in urine. Methylhippuric acid, the primary metabolite of xylenes, is formed from conjugation with glycine and oxidation of a methyl group. Xylenol is a minor metabolite obtained from aromatic hydroxylation of xylene. Other minor metabolites found in urine include methylbenzyl alcohol and glucuronic acid conjugates of the oxidized xylene. In humans, hepatic microsomal CYP2E1 is the primary enzyme involved in metabolisation of xylene to methylbenzylalcohol, intermediate in the methylhippuric acid pathway. Unmetabolized xylene is also found in urine, and can also be exhalated. (12) |
|---|
| Toxicity Values | LC50: 4740 ppm (Inhalation, Rat) (12)
LC50: 3907 ppm (Inhalation, Mouse) (12) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 3, not classifiable as to its carcinogenicity to humans. (11) |
|---|
| Uses/Sources | Xylene is used as a solvent and in the printing, rubber, and leather industries. It is also used as a cleaning agent, a thinner for paint, and in paints and varnishes. It is found in small amounts in airplane fuel and gasoline. Exposure to xylene may occur from breathing it in contaminated air, drinking or eating xylene-contaminated water or food, and through dermal and eye contact with xylene containing products. (12, 12) |
|---|
| Minimum Risk Level | Acute Inhalation: 2 ppm (12)
Intermediate Inhalation: 0.6 ppm (12)
Chronic Inhalation: 0.05 ppm (12)
Acute Oral: 1 mg/kg/day (12)
Intermediate Oral: 0.4 mg/kg/day (12)
Chronic Oral: 0.2 mg/kg/day (12) |
|---|
| Health Effects | Acute exposure to cholinesterase inhibitors can cause a cholinergic crisis characterized by severe nausea/vomiting, salivation, sweating, bradycardia, hypotension, collapse, and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved. Accumulation of ACh at motor nerves causes overstimulation of nicotinic expression at the neuromuscular junction. When this occurs symptoms such as muscle weakness, fatigue, muscle cramps, fasciculation, and paralysis can be seen. When there is an accumulation of ACh at autonomic ganglia this causes overstimulation of nicotinic expression in the sympathetic system. Symptoms associated with this are hypertension, and hypoglycemia. Overstimulation of nicotinic acetylcholine receptors in the central nervous system, due to accumulation of ACh, results in anxiety, headache, convulsions, ataxia, depression of respiration and circulation, tremor, general weakness, and potentially coma. When there is expression of muscarinic overstimulation due to excess acetylcholine at muscarinic acetylcholine receptors symptoms of visual disturbances, tightness in chest, wheezing due to bronchoconstriction, increased bronchial secretions, increased salivation, lacrimation, sweating, peristalsis, and urination can occur. Certain reproductive effects in fertility, growth, and development for males and females have been linked specifically to organophosphate pesticide exposure. Most of the research on reproductive effects has been conducted on farmers working with pesticides and insecticdes in rural areas. In females menstrual cycle disturbances, longer pregnancies, spontaneous abortions, stillbirths, and some developmental effects in offspring have been linked to organophosphate pesticide exposure. Prenatal exposure has been linked to impaired fetal growth and development. Neurotoxic effects have also been linked to poisoning with OP pesticides causing four neurotoxic effects in humans: cholinergic syndrome, intermediate syndrome, organophosphate-induced delayed polyneuropathy (OPIDP), and chronic organophosphate-induced neuropsychiatric disorder (COPIND). These syndromes result after acute and chronic exposure to OP pesticides. |
|---|
| Symptoms | Inhalation and ingestion can lead to dizziness, drowsiness, headache, and nausea. Burning sensations and abdominal pain can also result from ingestion. Dermal and eye exposure can cause skin dryness, redness, and pain. Conjunctivitis, dermatitis, respiratory tract irritation, dyspnea, anorexia, vomiting, fatigue, vertigo, incoordination, irritation, gangrene and anemia are other symptoms following xylene poisoning. (1) |
|---|
| Treatment | If the compound has been ingested, rapid gastric lavage should be performed using 5% sodium bicarbonate. For skin contact, the skin should be washed with soap and water. If the compound has entered the eyes, they should be washed with large quantities of isotonic saline or water. In serious cases, atropine and/or pralidoxime should be administered. Anti-cholinergic drugs work to counteract the effects of excess acetylcholine and reactivate AChE. Atropine can be used as an antidote in conjunction with pralidoxime or other pyridinium oximes (such as trimedoxime or obidoxime), though the use of '-oximes' has been found to be of no benefit, or possibly harmful, in at least two meta-analyses. Atropine is a muscarinic antagonist, and thus blocks the action of acetylcholine peripherally. |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB03463 |
|---|
| HMDB ID | HMDB59924 |
|---|
| PubChem Compound ID | 7809 |
|---|
| ChEMBL ID | CHEMBL31561 |
|---|
| ChemSpider ID | 7521 |
|---|
| KEGG ID | C06756 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 27417 |
|---|
| BioCyc ID | CPD-1422 |
|---|
| CTD ID | C031286 |
|---|
| Stitch ID | Xylene, para- |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 1806 |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Stephen Allan Butter, “Selective production of para-xylene.” U.S. Patent US4007231, issued September, 1971. |
|---|
| MSDS | T3D0185.pdf |
|---|
| General References | - Miller ER 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E: Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005 Jan 4;142(1):37-46. Epub 2004 Nov 10. [15537682 ]
- Grunder S, Valente C, Whalley AC, Sampath S, Portmann J, Botros YY, Stoddart JF: Molecular gauge blocks for building on the nanoscale. Chemistry. 2012 Dec 3;18(49):15632-49. doi: 10.1002/chem.201201985. Epub 2012 Oct 22. [23090871 ]
- Schneider CJ, Moubaraki B, Cashion JD, Turner DR, Leita BA, Batten SR, Murray KS: Spin crossover in di-, tri- and tetranuclear, mixed-ligand tris(pyrazolyl)methane iron(II) complexes. Dalton Trans. 2011 Jul 14;40(26):6939-51. doi: 10.1039/c0dt01725f. Epub 2011 Jun 6. [21643603 ]
- Latrache H, El GA, Karroua M, Hakkou A, Ait MH, El BA, Bourlioux P: Relations between hydrophobicity tested by three methods and surface chemical composition of Escherichia coli. New Microbiol. 2002 Jan;25(1):75-82. [11837394 ]
- Lyons TW, Guironnet D, Findlater M, Brookhart M: Synthesis of p-xylene from ethylene. J Am Chem Soc. 2012 Sep 26;134(38):15708-11. Epub 2012 Sep 13. [22934909 ]
- Grunder S, Stoddart JF: Giving substance to the Losanitsch series. Chem Commun (Camb). 2012 Mar 28;48(26):3158-60. doi: 10.1039/c2cc17734j. Epub 2012 Feb 16. [22343755 ]
- Svecova V, Topinka J, Solansky I, Sram RJ: Personal exposure to volatile organic compounds in the Czech Republic. J Expo Sci Environ Epidemiol. 2012 Sep;22(5):455-60. doi: 10.1038/jes.2012.30. Epub 2012 Jun 6. [22669500 ]
- Kirimura K, Nakagawa H, Tsuji K, Matsuda K, Kurane R, Usami S: Selective and continuous degradation of carbazole contained in petroleum oil by resting cells of Sphingomonas sp. CDH-7. Biosci Biotechnol Biochem. 1999 Sep;63(9):1563-8. [10540744 ]
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- ITII (1982). Toxic and Hazarous Industrial Chemicals Safety Manual. Tokyo, Japan: The International Technical Information Institute.
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2007). Toxicological profile for xylene. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- International Programme on Chemical Safety (IPCS) INCHEM (1992). Poison Information Monograph for Xylene. [Link]
- Wikipedia. P-Xylene. Last Updated 19 July 2009. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|