| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-03-06 18:58:16 UTC |
|---|
| Update Date | 2014-12-24 20:21:19 UTC |
|---|
| Accession Number | T3D0199 |
|---|
| Identification |
|---|
| Common Name | N-Nitrosodimethylamine |
|---|
| Class | Small Molecule |
|---|
| Description | N-Nitrosodimethylamine is found in pepper (Capsicum annuum). N-Nitrosodimethylamine is a food contaminant especially in cured meat products. N-Nitrosodimethylamine (NDMA), also known as dimethylnitrosamine (DMN), is a semi-volatile organic chemical that is highly toxic and is a suspected human carcinogen. The US Environmental Protection Agency has determined that the maximum admissible concentration of NDMA in drinking water is 7 ng L 1. The EPA has not yet set a regulatory maximum contaminant level (MCL) for drinking water. At high doses, it is a potent hepatotoxin that can cause fibrosis of the liver in rats. The induction of liver tumors in rats after chronic exposure to low doses is well-documented. Its toxic effects on humans are inferred from animal experiments but not well-established experimentally. NDMA is an industrial by-product or waste product of several industrial processes. It first came to attention as a groundwater contaminant in California in 1998 and 1999 at several sites that produced rocket fuel. Manufacturing of unsymmetrical dimethylhydrazine (UDMH), which is a component of rocket fuel that requires NDMA for its synthesis, proved to be the culprit in these cases. Of more general concern, water treatment via chlorination or chloramination of organic nitrogen-containing wastewater can lead to the production of NDMA at potentially harmful levels. Further, NDMA can form or be leached during treatment of water by anion exchange resins. Finally, NDMA is found at low levels in numerous items of human consumption including cured meat, fish, beer, and tobacco smoke It is, however, unlikely to bioaccumulate.
N-nitrosodimethylamine belongs to the family of Nitrosamines. These are compounds containing the nitrosamine functional group, with the structure R2NNO. |
|---|
| Compound Type | - Amine
- Cigarette Toxin
- Food Toxin
- Household Toxin
- Industrial By-product/Pollutant
- Industrial/Workplace Toxin
- Metabolite
- Nitrite
- Organic Compound
- Pesticide
- Pollutant
- Synthetic Compound
|
|---|
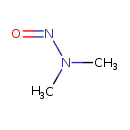
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1,1-Dimethyl-2-oxohydrazine | | Dimethyl-Nitrosamine | | Dimethylnitrosamin | | Dimethylnitrosamine | | Dimethylnitrosoamine | | DMNA | | N Nitrosodimethylamine | | N, N-Dimethylnitrosamine | | N,N-Dimethylnitrosamine | | N-Dimethyl-nitrosamine | | N-Dimethylnitrosoamine | | N-Methyl-N-nitroso-Methamine | | N-Methyl-N-nitroso-Methanamine | | N-Methyl-N-nitrosomethanamine | | N-Methyl-N-nitrosomethanamine, 9CI | | N-Nitroaodimethylamine | | N-Nitroso-Dimethylamine | | N-Nitroso-N,N-dimethylamine | | NDMA | | NDMA nitrosodimethylamine | | Nitrosodimethylamine | | Nitrous dimethylamide |
|
|---|
| Chemical Formula | C2H6N2O |
|---|
| Average Molecular Mass | 74.082 g/mol |
|---|
| Monoisotopic Mass | 74.048 g/mol |
|---|
| CAS Registry Number | 62-75-9 |
|---|
| IUPAC Name | dimethyl(nitroso)amine |
|---|
| Traditional Name | nitrosodimethylamine |
|---|
| SMILES | CN(C)N=O |
|---|
| InChI Identifier | InChI=1S/C2H6N2O/c1-4(2)3-5/h1-2H3 |
|---|
| InChI Key | InChIKey=UMFJAHHVKNCGLG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as organic n-nitroso compounds. These are organic compounds containing a n-nitroso group -NN=O. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Organic nitroso compounds |
|---|
| Direct Parent | Organic N-nitroso compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organic n-nitroso compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Yellow liquid. (6) |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | < 25°C | | Boiling Point | 154 °C | | Solubility | 1000 mg/mL at 24°C | | LogP | -0.57 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9000000000-6d29f9f7a8384c52f7b5 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-004i-9000000000-2df59bc19d1d15592467 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-004i-9000000000-744c0a10617e87137cd5 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-004i-9000000000-f3f3d5174fd1edee28a6 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-004i-9000000000-f3f3d5174fd1edee28a6 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-004i-9000000000-2df59bc19d1d15592467 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-004i-9000000000-4d289a54c5a2591daa33 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-004i-9000000000-5a8fb7bb4f334a3f8445 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-004i-9000000000-d0dbc7bf8508475d923d | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-004i-9000000000-744c0a10617e87137cd5 | 2021-09-20 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-9000000000-05b7b59e6e0b8b9fc245 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-9000000000-3910f1d3f8050d839f82 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004l-9000000000-4ab1e2e6522e0f1a2eb3 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-9000000000-eea50ce8e07045a0ad14 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9000000000-fed38e3c1ad8e9420fd6 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fr-9000000000-0d360c627861df90248b | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-9000000000-87753cf4baa848fb8803 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9000000000-87753cf4baa848fb8803 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fu-9000000000-fa476a49943ea98d47ff | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-9000000000-777dda7ebfc4febb2166 | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-9000000000-19ff9f5df3b310d929a0 | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002f-9000000000-876fe1a3967818d2717c | 2021-09-25 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-00dl-9000000000-25a83bcedddadb3e2b4e | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 25.16 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (6) ; inhalation (6) ; dermal (6) |
|---|
| Mechanism of Toxicity | The mechanism of NDMAinduced liver toxicity is not clearly understood but may be related to alkylation of cellular protein. (6) |
|---|
| Metabolism | Evidence from in vitro and in vivo studies with rodents indicates that NDMA is metabolized by hydroxylation of the alpha-carbon, followed by formation of formaldehyde, molecular nitrogen and a methylating agent, which is considered to be the carcinogenic form. Recent evidence suggests that a significant proportion of NDMA is metabolized via a denitrosation mechanism. (6) |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 2A, probably carcinogenic to humans. (7) |
|---|
| Uses/Sources | The general population might be exposed to NDMA from a wide variety of sources, including environmental, consumer, and occupational sources. The primary sources of human exposure to NDMA are tobacco smoke, chewing tobacco, diet (cured meats [particularly bacon], beer, fish, cheese, and other food items), toiletry and cosmetic products (for example, shampoos and cleansers), interior air of cars, and various other household goods, such as detergents and pesticides. In addition, NDMA can form in the stomach during digestion of alkylamine-containing foods. Alkylamines are naturally occurring compounds which are found in some drugs and in a variety of foods. Infants may be exposed to NDMA from the use of rubber baby bottle nipples and pacifiers which may contain very small amounts of NDMA, from ingestion of contaminated infant formulas, and from breast milk of some nursing mothers. Very low levels of NDMA have been found in some samples of human breast milk. Occupational exposure may happen in a large number of places including industries such as tanneries, pesticide manufacturing plants, rubber and tire manufacturing plants, alkylamine manufacture/use industries, fish processing industries, foundries, and dye manufacturing plants. Researchers making or handling NDMA may also be exposed to this compound if it passes through the rubber gloves they wear during laboratory work. (6) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | NDMA is very harmful to the liver of animals and humans. Moreover, although there are no reports of NDMA causing cancer in humans, it is reasonable to expect that exposure to NDMA by eating, drinking, or breathing could also cause cancer in humans. (6) |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB31419 |
|---|
| PubChem Compound ID | 6124 |
|---|
| ChEMBL ID | CHEMBL117311 |
|---|
| ChemSpider ID | 5894 |
|---|
| KEGG ID | C14704 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 35807 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | D004128 |
|---|
| Stitch ID | n-Nitrosodimethylamine |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 1050 |
|---|
| Wikipedia Link | N-Nitrosodimethylamine |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D0199.pdf |
|---|
| General References | - Godoy W, Albano RM, Moraes EG, Pinho PR, Nunes RA, Saito EH, Higa C, Filho IM, Kruel CD, Schirmer CC, Gurski R, Lang MA, Pinto LF: CYP2A6/2A7 and CYP2E1 expression in human oesophageal mucosa: regional and inter-individual variation in expression and relevance to nitrosamine metabolism. Carcinogenesis. 2002 Apr;23(4):611-6. [11960914 ]
- Lee MF, Liu ML, Cheng AC, Tsai ML, Ho CT, Liou WS, Pan MH: Pterostilbene inhibits dimethylnitrosamine-induced liver fibrosis in rats. Food Chem. 2013 Jun 1;138(2-3):802-7. doi: 10.1016/j.foodchem.2012.11.094. Epub 2012 Dec 1. [23411180 ]
- Lee MF, Tsai ML, Sun PP, Chien LL, Cheng AC, Ma NJ, Ho CT, Pan MH: Phyto-power dietary supplement potently inhibits dimethylnitrosamine-induced liver fibrosis in rats. Food Funct. 2013 Feb 26;4(3):470-5. doi: 10.1039/c2fo30306j. [23291610 ]
- Kao HW, Chen CL, Chang WY, Chen JT, Lin WJ, Liu RS, Wang HE: (18)F-FBHGal for asialoglycoprotein receptor imaging in a hepatic fibrosis mouse model. Bioorg Med Chem. 2013 Feb 15;21(4):912-21. doi: 10.1016/j.bmc.2012.12.022. Epub 2012 Dec 22. [23321012 ]
- Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
- ATSDR - Agency for Toxic Substances and Disease Registry (1989). Toxicological Profile for n-nitrosodimethylamine. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|