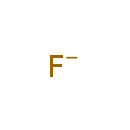| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-03-06 18:58:17 UTC |
|---|
| Update Date | 2014-12-24 20:21:20 UTC |
|---|
| Accession Number | T3D0211 |
|---|
| Identification |
|---|
| Common Name | Fluorine |
|---|
| Class | Small Molecule |
|---|
| Description | Fluorine (Latin: fluere, meaning to flow), is the chemical element with the symbol F and atomic number 9. It is a nonmetallic, diatomic gas that is a trace element and member of the halogen family. Pure fluorine (F2) is a corrosive, poisonous, pale yellowish brown gas that is a powerful oxidizing agent. It is the most reactive and electronegative of all the elements (4.0), and readily forms compounds with most other elements. Fluorine even combines with the noble gases, krypton, xenon, and radon. Even in dark, cool conditions, fluorine reacts explosively with hydrogen. It is so reactive that glass, metals, and even water, as well as other substances, burn with a bright flame in a jet of fluorine gas. It is far too reactive to be found in elemental form and has such an affinity for most elements, including silicon, that it can neither be prepared nor be kept in ordinary glass vessels. Instead, it must be kept in specialized quartz tubes lined with a very thin layer of fluorocarbons. In moist air it reacts with water to form also-dangerous hydrofluoric acid. Elemental fluorine is a powerful oxidizer which can cause organic material, combustibles, or other flammable materials to ignite. Both elemental fluorine and fluoride ions are highly toxic and must be handled with great care and any contact with skin and eyes should be strictly avoided. Physiologically, fluorine. exists as an ion in the body. When it is a free element, fluorine has a characteristic pungent odor that is detectable in concentrations as low as 20 nL/L. Fluorine is used in dentistry as flouride (Fluorides) to prevent dental caries. Sodium and stannous salts of fluorine are commonly used in dentifrices. Contact of exposed skin with HF (hydrofluoric acid) solutions posses one of the most extreme and insidious industrial threats-- one which is exacerbated by the fact that HF damages nerves in such a way as to make such burns initially painless. The HF molecule is capable of rapidly migrating through lipid layers of cells which would ordinarily stop an ionized acid, and the burns are typically deep. HF may react with calcium, permanently damaging the bone. More seriously, reaction with the body's calcium can cause cardiac arrhythmias, followed by cardiac arrest brought on by sudden chemical changes within the body. These cannot always be prevented with local or intravenous injection of calcium salts. HF spills over just 2.5% of the body's surface area, despite copious immediate washing, have been fatal If the patient survives, HF burns typically produce open wounds of an especially slow-healing nature. Fluorine in the form of fluorspar (also called fluorite) (calcium fluoride) was described in 1530 by Georgius Agricola for its use as a flux , which is a substance that is used to promote the fusion of metals or minerals. In 1670 Schwanhard found that glass was etched when it was exposed to fluorspar that was treated with acid. Karl Scheele and many later researchers, including Humphry Davy, Gay-Lussac, Antoine Lavoisier, and Louis Thenard all would experiment with hydrofluoric acid, easily obtained by treating calcium fluoride (fluorspar) with concentrated sulfuric acid. |
|---|
| Compound Type | - Food Toxin
- Halogen
- Industrial/Workplace Toxin
- Inorganic Compound
- Metabolite
- Natural Compound
- Non-Metal
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | F(-1) | | Fluoride | | Fluorine anion | | Fluorine(-1) anion |
|
|---|
| Chemical Formula | F |
|---|
| Average Molecular Mass | 18.999 g/mol |
|---|
| Monoisotopic Mass | 18.999 g/mol |
|---|
| CAS Registry Number | 7782-41-4 |
|---|
| IUPAC Name | fluoride |
|---|
| Traditional Name | fluoride |
|---|
| SMILES | [F-] |
|---|
| InChI Identifier | InChI=1S/FH/h1H/p-1 |
|---|
| InChI Key | InChIKey=KRHYYFGTRYWZRS-UHFFFAOYSA-M |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as homogeneous halogens. These are inorganic non-metallic compounds in which the largest atom is a nobel gas. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Homogeneous non-metal compounds |
|---|
| Class | Homogeneous halogens |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Homogeneous halogens |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | |
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cell surface
- Cytoplasm
- Extracellular
- Plasma Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | |
|---|
| Pathways | |
|---|
| Applications | Not Available |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Gas |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | -219.61°C | | Boiling Point | Not Available | | Solubility | 0.00169 mg/mL at 25°C | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-9000000000-15e49aa98d47f1ff8145 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9000000000-15e49aa98d47f1ff8145 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9000000000-15e49aa98d47f1ff8145 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-9000000000-0ca435743791c1006c74 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-9000000000-0ca435743791c1006c74 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-9000000000-0ca435743791c1006c74 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-9000000000-292905fbf11dc3acf140 | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-9000000000-292905fbf11dc3acf140 | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-9000000000-292905fbf11dc3acf140 | 2021-09-25 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (8) ; inhalation (8) ; dermal (8) |
|---|
| Mechanism of Toxicity | Fluoride ions are incorporated into bone by substituting for hydroxyl groups in the carbonate-apatite structure to produce hydroxyfluorapatite, thus altering the mineral structure of the bone. Alteration in mineralization increases hardness and bone mass, but also decreases mechanical strength. A portion of the circulating inorganic fluoride acts as an enzyme inhibitor because it forms metalfluoride-phosphate complexes that interfere with the activity of those enzymes requiring a metal ion cofactor. In addition, fluoride may interact directly with the enzyme or the substrate. It is a general inhibitor of the energy production system of the cell. Fluorine may bind calcium and decrease its concentration. This is thought to indirectly inhibit amelogeninase activity, resulting in altered crystal growth and subsequently causing dental fluorosis. (8) |
|---|
| Metabolism | Fluorides may be absorbed following inhalation, oral, or dermal exposure. Once in the body, the fluoride ion is transported in the blood and accumulates in the bones and teeth. Fluoride is believed to replace the hydroxyl ion (OH-) and possibly the bicarbonate ion (HCO3-) associated with hydroxyapatite—a mineral phase during formation of bone. The resultant material is hydroxyfluorapatite. Once absorbed, a portion of the fluoride is deposited in the skeleton, and most of the remainder is excreted in the urine, with smaller amounts in feces, and sweat, and saliva. (8) |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | 25 ppm over 5 minutes for an adult human. (7) |
|---|
| Carcinogenicity (IARC Classification) | Inorganic fluorides used in drinking-water are not classifiable as to their carcinogenicity to humans (Group 3). (11) |
|---|
| Uses/Sources | Fluoride compounds are used in making steel, chemicals, ceramics, lubricants, dyes, plastics, and pesticides. Fluorides are often added to drinking water supplies and to a variety of dental products, including toothpaste and mouth rinses, to prevent dental cavities. (8) |
|---|
| Minimum Risk Level | Acute Inhalation: 0.01 ppm (6) |
|---|
| Health Effects | Exposure to high levels of fluoride can result in denser bones. However, if exposure is high enough, these bones may be more fragile and brittle and there may be a greater risk of fracture. Chronic exposure may also cause dental fluorosis, which alters the appearance of children's teeth during tooth development. (8, 10) |
|---|
| Symptoms | Fluorine is very irritating to the skin, eyes, and respiratory tract. Symptoms of fluoride exposure include abdominal pain, diarrhea, dysphagia, hypersalivation, mucosal injury, nausea, vomiting. Electrolyte abnormalities including hyperkalemia, hypocalcemia, hypoglycemia, and hypomagnesemia may occur. Neurological symptoms include headache, muscle weakness, hyperactive reflexes, muscular spasms, paresthesia seizures, tetanic contractions, and tremors. In severe cases, multiorgan failure will occur. Death typically results from cardiac arrest, shock, widening of QRS, and various arrhythmias occur. (8, 10) |
|---|
| Treatment | Oral exposure to fluoride compounds should be treated by giving milk, calcium carbonate, or milk of magnesia to slow absorption. Eye or skin contact should be treated by removing any contaminated clothing and flushing with water. (10) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB00662 |
|---|
| PubChem Compound ID | 28179 |
|---|
| ChEMBL ID | CHEMBL1232767 |
|---|
| ChemSpider ID | 26214 |
|---|
| KEGG ID | C00742 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | 173395177400 |
|---|
| ChEBI ID | 17051 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | D005461 |
|---|
| Stitch ID | Fluorine |
|---|
| PDB ID | F |
|---|
| ACToR ID | 6447 |
|---|
| Wikipedia Link | Fluorine |
|---|
| References |
|---|
| Synthesis Reference | Li, Rong. Electrolytic cell for manufacturing fluorine at intermediate temperature. Shiyong Xinxing Zhuanli Shuomingshu (2007), 13pp. CODEN: CNXXAR CN 2895439 Y 20070502 CAN 147:310009 AN 2007:902560 |
|---|
| MSDS | Link |
|---|
| General References | - Schaafsma A, de Vries PJ, Saris WH: Delay of natural bone loss by higher intakes of specific minerals and vitamins. Crit Rev Food Sci Nutr. 2001 May;41(4):225-49. [11401244 ]
- Taylor A: Detection and monitoring of disorders of essential trace elements. Ann Clin Biochem. 1996 Nov;33 ( Pt 6):486-510. [8937580 ]
- Schneider E, Bolo NR, Frederick B, Wilkinson S, Hirashima F, Nassar L, Lyoo IK, Koch P, Jones S, Hwang J, Sung Y, Villafuerte RA, Maier G, Hsu R, Hashoian R, Renshaw PF: Magnetic resonance spectroscopy for measuring the biodistribution and in situ in vivo pharmacokinetics of fluorinated compounds: validation using an investigation of liver and heart disposition of tecastemizole. J Clin Pharm Ther. 2006 Jun;31(3):261-73. [16789992 ]
- Thie JA, Smith GT, Hubner KF: 2-deoxy-2-[F-18]fluoro-D-glucose-positron emission tomography sensitivity to serum glucose: a survey and diagnostic applications. Mol Imaging Biol. 2005 Sep-Oct;7(5):361-8. [16228119 ]
- McGoron AJ, Mao X, Georgiou MF, Kuluz JW: Computer phantom study of brain PET glucose metabolism imaging using a rotating SPECT/PET camera. Comput Biol Med. 2005 Jul;35(6):511-31. [15780862 ]
- ATSDR - Agency for Toxic Substances and Disease Registry (2001). Minimal Risk Levels (MRLs) for Hazardous Substances. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- HSDB: Hazardous Substances Data Bank. National Library of Medicine (2001). [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2003). Toxicological profile for fluorides, hydrogen fluoride, and fluorine. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Fluorine. Last Updated 27 June 2009. [Link]
- Wikipedia. Fluoride poisoning. Last Updated 19 May 2009. [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|