| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-03-06 18:58:18 UTC |
|---|
| Update Date | 2014-12-24 20:21:21 UTC |
|---|
| Accession Number | T3D0216 |
|---|
| Identification |
|---|
| Common Name | Nitrate |
|---|
| Class | Small Molecule |
|---|
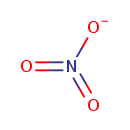
| Description | In inorganic chemistry, a nitrate is a salt of nitric acid. In organic chemistry the esters of nitric acid and various alcohols are called nitrates. The nitrate ion is a polyatomic anion with the empirical formula NO3- and a molecular mass of 62.01 daltons; it consists of one central nitrogen atom surrounded by three identical oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a negative one formal charge. Nitrates should not be confused with nitrites, the salts of nitrous acid. Organic compounds containing the nitro functional group (which has the same formula and structure as the nitrate ion save that one of the O2 atoms is replaced by the R group) are known as nitro compounds. Nitrate ions can be toxic. In particular, nitrate toxicosis in humans occurs through enterohepatic metabolism of nitrates to ammonia, with nitrite being an intermediate. Nitrites oxidize the iron atoms in hemoglobin from Ferrous Iron (2+) to Ferric Iron (3+), rendering it unable to carry oxygen. This condition is called methemoglobinemia and can lead to a lack of oxygen in tissues. Methemoglobinemia can be treated with methylene blue. -- Wikipedia. |
|---|
| Compound Type | - Food Toxin
- Household Toxin
- Inorganic Compound
- Metabolite
- Natural Compound
- Nitrate
- Nitrite
- Non-Metal
- Pesticide
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Nitrate ion | | Nitrate(1-) | | Nitric acid | | Trioxidonitrate | | Trioxidonitrate(1-) | | Trioxonitrate | | Trioxonitrate(1-) |
|
|---|
| Chemical Formula | NO3 |
|---|
| Average Molecular Mass | 62.005 g/mol |
|---|
| Monoisotopic Mass | 61.988 g/mol |
|---|
| CAS Registry Number | 14797-55-8 |
|---|
| IUPAC Name | nitrate |
|---|
| Traditional Name | nitrate |
|---|
| SMILES | [O-]N(=O)=O |
|---|
| InChI Identifier | InChI=1S/NO3/c2-1(3)4/q-1 |
|---|
| InChI Key | InChIKey=NHNBFGGVMKEFGY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as non-metal nitrates. These are inorganic non-metallic compounds containing a nitrate as its largest oxoanion. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Homogeneous non-metal compounds |
|---|
| Class | Non-metal oxoanionic compounds |
|---|
| Sub Class | Non-metal nitrates |
|---|
| Direct Parent | Non-metal nitrates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Non-metal nitrate
- Inorganic oxide
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | - Adipose Tissue
- Bladder
- Epidermis
- Fibroblasts
- Heart
- Intestine
- Kidney
- Lung
- Myelin
- Nerve Cells
- Neuron
- Pancreas
- Platelet
- Skeletal Muscle
- Spleen
- Stratum Corneum
- Testes
- Thyroid Gland
|
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 73°C (163.4°F) | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Negative (Annotated) | splash10-03di-9000000000-62b6946f773f2794ce2b | 2012-07-25 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Negative (Annotated) | splash10-03dj-9000000000-b705d64f017cd9970bcb | 2012-07-25 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Negative (Annotated) | splash10-03di-9000000000-a03d650125e7c3c9631a | 2012-07-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-9000000000-6d9d3ad97d4b8f473d63 | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-9000000000-30aa91b9c1649e1a8e72 | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-9000000000-8ec454185b5a5da7b125 | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03dj-9000000000-4de59f98968f9eaff499 | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-9000000000-272e3456c62a5f032c8c | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-9000000000-2325ea6feac64f6d9cbd | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-9000000000-8c9248f9c4b7a86e3459 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-9000000000-8c9248f9c4b7a86e3459 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-9000000000-8c9248f9c4b7a86e3459 | 2021-09-22 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (18) ; inhalation (18) |
|---|
| Mechanism of Toxicity | Nitrate's toxicity is a result of it's conversion to nitrite once in the body. Nitrite causes the autocatalytic oxidation of oxyhemoglobin to hydrogen peroxide and methemoglobin. This elevation of methemoglobin levels is a condition known as methemoglobinemia, and is characterized by tissue hypoxia, as methemoglobin cannot bind oxygen. (1, 19) |
|---|
| Metabolism | Intake of some amount of nitrates and nitrites is a normal part of the nitrogen cycle in humans. In vivo conversion of nitrates to nitrites can occur in the gastrointestional tract under the right conditions, significantly enhancing nitrates' toxic potency. The major metabolic pathway for nitrate is conversion to nitrite, and then to ammonia. Nitrites, nitrates, and their metabolites are excreted in the urine. (18) |
|---|
| Toxicity Values | LD50: 138 ppm over 30 minutes (Inhalation, Rat) (2) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Ingested nitrate or nitrite under conditions that result in endogenous nitrosation is probably carcinogenic to humans (Group 2A). (17) |
|---|
| Uses/Sources | Nitrates and nitrites are naturally produced and may also be found in pesticides. Exposure usually occurs from contact with contaminated soil, food water. Nitrates may also be found in certain drugs. (18) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Nitrate and nitrite poisoning causes methemoglobinemia. Nitrites may cause pregnancy complications and developmental effects. They may also be carcinogenic. (18) |
|---|
| Symptoms | Nitrate and nitrite poisoning causes methemoglobinemia. Symptoms include cyanosis, cardiac dysrhythmias and circulatory failure, and progressive central nervous system (CNS) effects. CNS effects can range from mild dizziness and lethargy to coma and convulsions. (18) |
|---|
| Treatment | Methemoglobinemia can be treated with supplemental oxygen and methylene blue 1% solution administered intravenously slowly over five minutes followed by IV flush with normal saline. Methylene blue restores the iron in hemoglobin to its normal (reduced) oxygen-carrying state. (19) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB02878 |
|---|
| PubChem Compound ID | 943 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 918 |
|---|
| KEGG ID | C00244 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | 109270 , 125853 , 163729 |
|---|
| ChEBI ID | 17632 |
|---|
| BioCyc ID | CPD-144 |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Nitrate |
|---|
| PDB ID | NO3 |
|---|
| ACToR ID | 6494 |
|---|
| Wikipedia Link | Nitrate |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Link |
|---|
| General References | - Keszler A, Piknova B, Schechter AN, Hogg N: The reaction between nitrite and oxyhemoglobin: a mechanistic study. J Biol Chem. 2008 Apr 11;283(15):9615-22. doi: 10.1074/jbc.M705630200. Epub 2008 Jan 17. [18203719 ]
- Park CH, Carboni E, Wood PL, Gee KW: Characterization of peripheral benzodiazepine type sites in a cultured murine BV-2 microglial cell line. Glia. 1996 Jan;16(1):65-70. [8787774 ]
- Rachid MA, Camargos ER, Barcellos L, Marques CA, Chiari E, Huang H, Tanowitz HB, Teixeira MM, Machado CR: Blockade of endothelin ET(A)/ET(B) receptors favors a role for endothelin during acute Trypanosoma cruzi infection in rats. Microbes Infect. 2006 Jul;8(8):2113-9. Epub 2006 Jun 6. [16844401 ]
- Susilo R, Korting HC, Strauss UP, Menke G, Schuster O, Menke A: Rate and extent of percutaneous absorption of sertaconazole nitrate after topical administration. Arzneimittelforschung. 2005;55(6):338-42. [16032974 ]
- Miyado T, Tanaka Y, Nagai H, Takeda S, Saito K, Fukushi K, Yoshida Y, Wakida S, Niki E: Simultaneous determination of nitrate and nitrite in biological fluids by capillary electrophoresis and preliminary study on their determination by microchip capillary electrophoresis. J Chromatogr A. 2004 Oct 8;1051(1-2):185-91. [15532572 ]
- Mitsui T, Kondo T: Assessing nitrate metabolism in the intestinal tract by measuring breath nitric oxide and nitrous oxide, and its clinical significance. Clin Chim Acta. 2002 May 7;319(1):57-62. [11922924 ]
- Boll MC, Alcaraz-Zubeldia M, Montes S, Murillo-Bonilla L, Rios C: Raised nitrate concentration and low SOD activity in the CSF of sporadic ALS patients. Neurochem Res. 2003 May;28(5):699-703. [12716019 ]
- Goncalves J, Wasif N, Esposito D, Coico JM, Schwartz B, Higgins PJ, Bockman RS, Staiano-Coico L: Gallium nitrate accelerates partial thickness wound repair and alters keratinocyte integrin expression to favor a motile phenotype. J Surg Res. 2002 Apr;103(2):134-40. [11922726 ]
- Ceregrzyn M, Kamata T, Yajima T, Kuwahara A: Biphasic alterations in gastrointestinal transit following endotoxaemia in mice. Neurogastroenterol Motil. 2001 Dec;13(6):605-13. [11903922 ]
- Bories C, Scherman E, Bories PN: Serum and tissue nitrate levels in murine visceral leishmaniasis correlate with parasite load but not with host protection. Trans R Soc Trop Med Hyg. 1997 Jul-Aug;91(4):433-6. [9373644 ]
- Parslow RC, McKinney PA, Law GR, Staines A, Williams R, Bodansky HJ: Incidence of childhood diabetes mellitus in Yorkshire, northern England, is associated with nitrate in drinking water: an ecological analysis. Diabetologia. 1997 May;40(5):550-6. [9165223 ]
- Tajtakova M, Langer P, Semanova Z, Tomkova Z, Szokeova E, Majoros J, Petrovicova J, Veseliny E: [Nitrate contaminated drinking water from private wells has an impact on the size and functional state of the thyroid gland in schoolchildren]. Vnitr Lek. 2000 Nov;46(11):764-7. [15637891 ]
- Weyer PJ, Cerhan JR, Kross BC, Hallberg GR, Kantamneni J, Breuer G, Jones MP, Zheng W, Lynch CF: Municipal drinking water nitrate level and cancer risk in older women: the Iowa Women's Health Study. Epidemiology. 2001 May;12(3):327-38. [11338313 ]
- Mukhopadhyay S, Ghosh D, Chatterjee A, Sinha S, Tripathy S, Chandra AK: Evaluation of possible goitrogenic and anti-thyroidal effect of nitrate, a potential environmental pollutant. Indian J Physiol Pharmacol. 2005 Jul-Sep;49(3):284-8. [16440845 ]
- Tsikas D: Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic Res. 2005 Aug;39(8):797-815. [16036360 ]
- Environment Canada (1981). Tech Info for Problem Spills: Nitric acid.
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2007). Case Studies in Environmental Medicine. Nitrate/Nitrite Toxicity. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Methemoglobinemia. Last Updated 22 July 2009. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (1997). Toxicological profile for hydrazine. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Nitrate. Last Updated 15 August 2014. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|