| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-03-06 18:58:25 UTC |
|---|
| Update Date | 2014-12-24 20:21:26 UTC |
|---|
| Accession Number | T3D0271 |
|---|
| Identification |
|---|
| Common Name | Styrene |
|---|
| Class | Small Molecule |
|---|
| Description | Styrene is found in alcoholic beverages. Styrene is present in cranberry, bilberry, currants, grapes, vinegar, parsley, milk and dairy products, whisky, cocoa, coffee, tea, roasted filberts and peanuts. Styrene is a flavouring ingredient. Polymers are used in ion-exchange resins in food processing. Indirect food additive arising from adhesives, oatings and packaging materials. Styrene, also known as vinyl benzene, is a colorless oily liquid that evaporates easily and has a sweet smell, although high concentrations confer a less pleasant odor. Styrene is the precursor to polystyrene and several copolymers. Low levels of styrene occur naturally in plants as well as a variety of foods such as fruits, vegetables, nuts, beverages, and meats. (Wikipedia)
Styrene has been shown to exhibit signalling and catabolic functions (1, 2).
Styrene belongs to the family of Styrenes. These are organic compounds containing an ethenylbenzene moiety. |
|---|
| Compound Type | - Aromatic Hydrocarbon
- Cigarette Toxin
- Food Toxin
- Household Toxin
- Industrial Precursor/Intermediate
- Industrial/Workplace Toxin
- Metabolite
- Natural Compound
- Organic Compound
- Pollutant
|
|---|
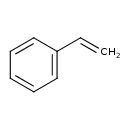
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Cinnamene | | Cinnamenol | | Cinnaminol | | Cinnamol | | Ethenyl-Benzene | | Ethenylbenzene | | Phenethylene | | Phenyl-Ethylene | | Phenylethene | | Phenylethylene | | Styrol | | Styrolene | | Vinyl benzene | | Vinyl-Benzene | | Vinylbenzene | | Vinylbenzol |
|
|---|
| Chemical Formula | C8H8 |
|---|
| Average Molecular Mass | 104.149 g/mol |
|---|
| Monoisotopic Mass | 104.063 g/mol |
|---|
| CAS Registry Number | 100-42-5 |
|---|
| IUPAC Name | ethenylbenzene |
|---|
| Traditional Name | styrene |
|---|
| SMILES | C=CC1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C8H8/c1-2-8-6-4-3-5-7-8/h2-7H,1H2 |
|---|
| InChI Key | InChIKey=PPBRXRYQALVLMV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as styrenes. These are organic compounds containing an ethenylbenzene moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Styrenes |
|---|
| Direct Parent | Styrenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Styrene
- Aromatic hydrocarbon
- Cyclic olefin
- Unsaturated hydrocarbon
- Olefin
- Hydrocarbon
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Colorless liquid. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | -33°C | | Boiling Point | Not Available | | Solubility | 0.31 mg/mL at 25°C | | LogP | 2.95 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - CI-B (Non-derivatized) | splash10-0a4i-0900000000-582bcbfa5c258cd958c7 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - CI-B (Non-derivatized) | splash10-0a4i-0900000000-582bcbfa5c258cd958c7 | 2018-05-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-9700000000-fcc077c4a70991516aa2 | 2016-09-22 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-000i-9000000000-65bc7025d0f253326456 | 2020-07-21 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 10V, positive | splash10-004i-9100000000-5280fafb1a35e353dc3d | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 30V, positive | splash10-004i-9200000000-872c7b6ee7f7a539f7ac | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 50V, positive | splash10-004i-9200000000-f1c3135a0df129e8ec5f | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 75V, positive | splash10-0fb9-9100000000-c41887824ab40bd09676 | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 100V, positive | splash10-0ufr-9000000000-a08dcd64cf252eac173d | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 125V, positive | splash10-0ufr-9000000000-6d6f3165b72ff298647c | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 150V, positive | splash10-0ufr-9000000000-af157200fe186aee0519 | 2020-07-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0900000000-257f37595a29cd4d0516 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1900000000-29144f6013919212fc12 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-056r-9300000000-55e21db93c2a93dcb229 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-93a8c8c37d30fea4dc59 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0900000000-1346486826c15e466ac6 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-6900000000-9cf38d14243780fea0da | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0900000000-d9a37da852aa04958c50 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-9600000000-b66c0ed4c5a2ec64853d | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-9000000000-b463cbf4ba16bde190ec | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-138b8c24fd394024a31a | 2021-09-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0900000000-138b8c24fd394024a31a | 2021-09-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-73515b4a2a95628c8b69 | 2021-09-24 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0udi-9800000000-1fdcf83a0134198725e5 | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 90 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 15.09 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (9) ; Inhalation (9) ; Dermal (9) |
|---|
| Mechanism of Toxicity | Styrene 7,8-oxide, a metabolite of styrene, can form DNA adducts by binding to deoxyguanosine. It is also mutagenic and causes increased frequency of sister chromatid exchange, chromosomal aberrations, micronucleated cells, and DNA strand breaks. (8) |
|---|
| Metabolism | Styrene may be absorbed following ingestion, inhalation, or dermal exposure. It distributes throughout the body in the blood, concentrating in the adipose tissue, kidney, and liver. The primary metabolic pathway is oxidation of the side chain by cytochrome P450 to form styrene 7,8-oxide. Styrene oxide is predominantly metabolized by epoxide hydrolase to form styrene glycol; the styrene glycol is subsequently converted to mandelic acid, phenylglyoxylic acid, and hippuric acid. Styrene 7,8-oxide can also be conjugated with glutathione to ultimately form phenylhydroxylethylmercapturic acids. A minor pathway of styrene metabolism involves the formation of phenylacetaldehyde from styrene 7,8-oxide or cytochrome P450 conversion of styrene to pheylethanol and subsequent metabolism to phenylacetic acid. An alternative minor pathway involves ring oxidation resulting in the production of styrene 3,4-oxide, which is further metabolized to 4-vinylphenol. The metabolites of styrene are excreted mainly in the urine. (4) |
|---|
| Toxicity Values | LD50: 316 mg/kg (Oral, Mouse) (3)
LD50: 898 mg/kg (Intraperitoneal, Rat) (3)
LC50: 24 g/m3 over 4 hours (Inhalation, Rat) (3) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 2B, possibly carcinogenic to humans. (7) |
|---|
| Uses/Sources | Styrene is used to make plastics and rubber. It is a precursor to polystyrene and may be found in insulation, fiberglass, plastic pipes, automobile parts, shoes, drinking cups and other food containers, and carpet backing. Low levels of styrene also occur naturally in a variety of foods such as fruits, vegetables, nuts, beverages, and meats. Small amounts of styrene can be transferred to food from styrene-based packaging material. (8) |
|---|
| Minimum Risk Level | Acute Inhalation: 2 ppm (6)
Chronic Inhalation: 0.2 ppm (6)
Acute Oral: 0.1 mg/kg/day (6) |
|---|
| Health Effects | Styrene causes nervous system depression and may be carcinogenic. Animals studies have also shown that hearing loss and liver damage may occur. (8, 9) |
|---|
| Symptoms | Breathing high levels of styrene may cause nervous system effects such as changes in color vision, tiredness, feeling drunk, slowed reaction time, concentration problems, or balance problems. Chest burning, wheezing, and dyspnea may also occur. Styrene is irritating to the skin, eyes, and respiratory tract. (8, 9) |
|---|
| Treatment | Treatment is mainly symptomatic and supportive. Respiratory assistance may be needed. (9) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB34240 |
|---|
| PubChem Compound ID | 7501 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 7220 |
|---|
| KEGG ID | C07083 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 27452 |
|---|
| BioCyc ID | CPD-1075 |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Styrene |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Styrene |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D0271.pdf |
|---|
| General References | - Roder-Stolinski C, Fischader G, Oostingh GJ, Feltens R, Kohse F, von Bergen M, Morbt N, Eder K, Duschl A, Lehmann I: Styrene induces an inflammatory response in human lung epithelial cells via oxidative stress and NF-kappaB activation. Toxicol Appl Pharmacol. 2008 Sep 1;231(2):241-7. doi: 10.1016/j.taap.2008.04.010. Epub 2008 Apr 29. [18554678 ]
- Alonso S, Bartolome-Martin D, del Alamo M, Diaz E, Garcia JL, Perera J: Genetic characterization of the styrene lower catabolic pathway of Pseudomonas sp. strain Y2. Gene. 2003 Nov 13;319:71-83. [14597173 ]
- Lewis RJ (1996). Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold.
- USEPA (1980). Ambient Water Quality Criteria Doc: Dinitrotoluene. EPA 440/5-80-045.
- Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
- ATSDR - Agency for Toxic Substances and Disease Registry (2001). Minimal Risk Levels (MRLs) for Hazardous Substances. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2007). Toxicological Profile for styrene. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- International Programme on Chemical Safety (IPCS) INCHEM (1996). Poison Information Monograph for Styrene. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|