Cobalt(II) nitrate (T3D0665)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-03-22 20:55:34 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:22:30 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D0665 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Cobalt(II) nitrate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Cobalt(II) nitrate is a nitrate of cobalt. The high solubility of cobalt nitrate makes it a common source of cobalt in metal-organic frameworks and polymers. It is also reduced to metallic cobalt or precipitated on various substrates for Fischer-Tropsch catalysis. Cobalt is a metallic element with the atomic number 27. It is found naturally in rocks, soil, water, plants, and animals. In small amounts cobalt is an essential element for life, as it is part of vitamin B12. However, excess exposure is known to exhibit toxic effects. Nitrite is a toxic compound known to cause methemoglobinemia. (10, 4, 5, 9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
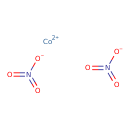
| Chemical Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | CoN2O6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 182.943 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 182.909 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 10141-05-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | λ²-cobalt(2+) ion dinitrate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | λ²-cobalt(2+) ion dinitrate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | [Co++].[O-]N(=O)=O.[O-]N(=O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/Co.2NO3/c;2*2-1(3)4/q+2;2*-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=UFMZWBIQTDUYBN-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of inorganic compounds known as transition metal nitrates. These are inorganic compounds in which the largest oxoanion is nitrate, and in which the heaviest atom not in an oxoanion is a transition metal. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Inorganic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Mixed metal/non-metal compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Transition metal oxoanionic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Transition metal nitrates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Transition metal nitrates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | Red solid. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Inhalation (4) ; oral (4) ; dermal (4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Cobalt is believed to exhibit its toxicity through a oxidant-based and free radical-based processes. It produces oxygen radicals and may be oxidized to ionic cobalt, causing increased lipid peroxidation, DNA damage, and inducing certain enzymes that lead to cell apoptosis. Cobalt has also been shown to block inorganic calcium channels, possibly impairing neurotransmission. Cobalt can also chelate lipoic acids, impairing oxidation of pyruvate or fatty acids. In addition, cobalt may inhibit DNA repair by interacting with zinc finger DNA repair proteins, and has also been shown to inhibit heme synthesis and glucose metabolism. Cobalt may activate specific helper T-lymphocyte cells and interact directly with immunologic proteins, such as antibodies (IgA and IgE) or Fc receptors, resulting in immunosensitization. (4) Nitrate's toxicity is a result of it's conversion to nitrite once in the body. Nitrite causes the autocatalytic oxidation of oxyhemoglobin to hydrogen peroxide and methemoglobin. This elevation of methemoglobin levels is a condition known as methemoglobinemia, and is characterized by tissue hypoxia, as methemoglobin cannot bind oxygen. (3, 11) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Cobalt is absorbed though the lungs, gastrointestinal tract, and skin. Since it is a component of the vitamin B12 (cyanocobalamin), it is distributed to most tissues of the body. It is transported in the blood, often bound to albumin, with the highest levels being found in the liver and kidney. Cobalt is excreted mainly in the urine and faeces. Intake of some amount of nitrates and nitrites is a normal part of the nitrogen cycle in humans. In vivo conversion of nitrates to nitrites can occur in the gastrointestional tract under the right conditions, significantly enhancing nitrates' toxic potency. The major metabolic pathway for nitrate is conversion to nitrite, and then to ammonia. Nitrites, nitrates, and their metabolites are excreted in the urine. (10, 4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | LD50: 434 mg/kg (Oral, Rat) (2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | Ingested nitrate or nitrite under conditions that result in endogenous nitrosation is probably carcinogenic to humans (Group 2A). Soluble cobalt(II) salts are possibly carcinogenic to humans (Group 2B). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | The high solubility of cobalt nitrate makes it a common source of cobalt in metal-organic frameworks and polymers. It is also reduced to metallic cobalt or precipitated on various substrates for Fischer-Tropsch catalysis. (9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Chronic Inhalation: 0.0001 mg/m3 (6) Intermediate Oral: 0.01 mg/kg/day (6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Exposure to high amount of cobalt can cause heart, lung, kidney, and liver damage. Skin contact is known to result in contact dermatitus. Cobalt may also have mutagenic and carcinogenic effects. Nitrate and nitrite poisoning causes methemoglobinemia. Nitrites may cause pregnancy complications and developmental effects. They may also be carcinogenic. (10, 4, 5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Cobalt inhalation can cause asthma-like breathing problems. Skin contact is known to result in contact dermatitis, which is characterized by irritation and rashes. Ingesting large amounts of cobalt may cause nausea and vomiting. Nitrate and nitrite poisoning causes methemoglobinemia. Symptoms include cyanosis, cardiac dysrhythmias and circulatory failure, and progressive central nervous system (CNS) effects. CNS effects can range from mild dizziness and lethargy to coma and convulsions. (12, 10, 4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Treatment of cobalt poisoning is symptomatic. Methemoglobinemia can be treated with supplemental oxygen and methylene blue 1% solution administered intravenously slowly over five minutes followed by IV flush with normal saline. Methylene blue restores the iron in hemoglobin to its normal (reduced) oxygen-carrying state. (11, 4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 25000 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 23369 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | C025913 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Cobalt(II) nitrate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | 12408 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Cobalt(II)_nitrate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | T3D0665.pdf | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Zinc ion binding
- Specific Function:
- Reversible hydration of carbon dioxide. Can hydrates cyanamide to urea.
- Gene Name:
- CA1
- Uniprot ID:
- P00915
- Molecular Weight:
- 28870.0 Da
References
- Ekinci D, Beydemir S, Kufrevioglu OI: In vitro inhibitory effects of some heavy metals on human erythrocyte carbonic anhydrases. J Enzyme Inhib Med Chem. 2007 Dec;22(6):745-50. doi: 10.1080/14756360601176048 . [18237030 ]
- Ul-Hassan M, Scozzafava A, Chohan ZH, Supuran CT: Carbonic anhydrase inhibitors: metal complexes of a sulfanilamide derived Schiff base and their interaction with isozymes I, II and IV. J Enzyme Inhib. 2001 Dec;16(6):499-505. [12164389 ]
- General Function:
- Zinc ion binding
- Specific Function:
- Essential for bone resorption and osteoclast differentiation (By similarity). Reversible hydration of carbon dioxide. Can hydrate cyanamide to urea. Involved in the regulation of fluid secretion into the anterior chamber of the eye. Contributes to intracellular pH regulation in the duodenal upper villous epithelium during proton-coupled peptide absorption. Stimulates the chloride-bicarbonate exchange activity of SLC26A6.
- Gene Name:
- CA2
- Uniprot ID:
- P00918
- Molecular Weight:
- 29245.895 Da
References
- Ekinci D, Beydemir S, Kufrevioglu OI: In vitro inhibitory effects of some heavy metals on human erythrocyte carbonic anhydrases. J Enzyme Inhib Med Chem. 2007 Dec;22(6):745-50. doi: 10.1080/14756360601176048 . [18237030 ]
- Ul-Hassan M, Scozzafava A, Chohan ZH, Supuran CT: Carbonic anhydrase inhibitors: metal complexes of a sulfanilamide derived Schiff base and their interaction with isozymes I, II and IV. J Enzyme Inhib. 2001 Dec;16(6):499-505. [12164389 ]
- General Function:
- Zinc ion binding
- Specific Function:
- Reversible hydration of carbon dioxide. May stimulate the sodium/bicarbonate transporter activity of SLC4A4 that acts in pH homeostasis. It is essential for acid overload removal from the retina and retina epithelium, and acid release in the choriocapillaris in the choroid.
- Gene Name:
- CA4
- Uniprot ID:
- P22748
- Molecular Weight:
- 35032.075 Da
References
- Ekinci D, Beydemir S, Kufrevioglu OI: In vitro inhibitory effects of some heavy metals on human erythrocyte carbonic anhydrases. J Enzyme Inhib Med Chem. 2007 Dec;22(6):745-50. doi: 10.1080/14756360601176048 . [18237030 ]
- Ul-Hassan M, Scozzafava A, Chohan ZH, Supuran CT: Carbonic anhydrase inhibitors: metal complexes of a sulfanilamide derived Schiff base and their interaction with isozymes I, II and IV. J Enzyme Inhib. 2001 Dec;16(6):499-505. [12164389 ]
- General Function:
- Oxygen transporter activity
- Specific Function:
- Involved in oxygen transport from the lung to the various peripheral tissues.
- Gene Name:
- HBA1
- Uniprot ID:
- P69905
- Molecular Weight:
- 15257.405 Da
References
- General Function:
- Oxygen transporter activity
- Specific Function:
- Involved in oxygen transport from the lung to the various peripheral tissues.LVV-hemorphin-7 potentiates the activity of bradykinin, causing a decrease in blood pressure.Spinorphin: functions as an endogenous inhibitor of enkephalin-degrading enzymes such as DPP3, and as a selective antagonist of the P2RX3 receptor which is involved in pain signaling, these properties implicate it as a regulator of pain and inflammation.
- Gene Name:
- HBB
- Uniprot ID:
- P68871
- Molecular Weight:
- 15998.34 Da
References
- General Function:
- Oxygen transporter activity
- Specific Function:
- Involved in oxygen transport from the lung to the various peripheral tissues.
- Gene Name:
- HBD
- Uniprot ID:
- P02042
- Molecular Weight:
- 16055.41 Da
References
- General Function:
- Oxygen transporter activity
- Specific Function:
- The epsilon chain is a beta-type chain of early mammalian embryonic hemoglobin.
- Gene Name:
- HBE1
- Uniprot ID:
- P02100
- Molecular Weight:
- 16202.71 Da
References
- General Function:
- Oxygen transporter activity
- Specific Function:
- Gamma chains make up the fetal hemoglobin F, in combination with alpha chains.
- Gene Name:
- HBG1
- Uniprot ID:
- P69891
- Molecular Weight:
- 16140.37 Da
References
- General Function:
- Gamma chains make up the fetal hemoglobin F, in combination with alpha chains.
- Specific Function:
- Heme binding
- Gene Name:
- HBG2
- Uniprot ID:
- P69892
- Molecular Weight:
- 16126.35 Da
References
- General Function:
- Oxygen transporter activity
- Specific Function:
- Not Available
- Gene Name:
- HBM
- Uniprot ID:
- Q6B0K9
- Molecular Weight:
- 15617.97 Da
References
- General Function:
- Oxygen transporter activity
- Specific Function:
- Not Available
- Gene Name:
- HBQ1
- Uniprot ID:
- P09105
- Molecular Weight:
- 15507.575 Da
References
- General Function:
- Oxygen transporter activity
- Specific Function:
- The zeta chain is an alpha-type chain of mammalian embryonic hemoglobin.
- Gene Name:
- HBZ
- Uniprot ID:
- P02008
- Molecular Weight:
- 15636.845 Da
References
15. DNA
- General Function:
- Used for biological information storage.
- Specific Function:
- DNA contains the instructions needed for an organism to develop, survive and reproduce.
- Molecular Weight:
- 2.15 x 1012 Da
References
- ATSDR - Agency for Toxic Substances and Disease Registry (2004). Toxicological profile for cobalt. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- General Function:
- Protein homodimerization activity
- Specific Function:
- Involved in DNA excision repair. Initiates repair by binding to damaged sites with various affinities, depending on the photoproduct and the transcriptional state of the region. Required for UV-induced CHEK1 phosphorylation and the recruitment of CEP164 to cyclobutane pyrimidine dimmers (CPD), sites of DNA damage after UV irradiation.
- Gene Name:
- XPA
- Uniprot ID:
- P23025
- Molecular Weight:
- 31367.71 Da
References
- Hartwig A, Asmuss M, Ehleben I, Herzer U, Kostelac D, Pelzer A, Schwerdtle T, Burkle A: Interference by toxic metal ions with DNA repair processes and cell cycle control: molecular mechanisms. Environ Health Perspect. 2002 Oct;110 Suppl 5:797-9. [12426134 ]
- General Function:
- Antigen binding
- Specific Function:
- Ig alpha is the major immunoglobulin class in body secretions. It may serve both to defend against local infection and to prevent access of foreign antigens to the general immunologic system.
- Gene Name:
- IGHA1
- Uniprot ID:
- P01876
- Molecular Weight:
- 37654.29 Da
References
- Bencko V, Wagner V, Wagnerova M, Reichrtova E: Immuno-biochemical findings in groups of individuals occupationally and non-occupationally exposed to emissions containing nickel and cobalt. J Hyg Epidemiol Microbiol Immunol. 1983;27(4):387-94. [6663071 ]
- General Function:
- Antigen binding
- Specific Function:
- Ig alpha is the major immunoglobulin class in body secretions. It may serve both to defend against local infection and to prevent access of foreign antigens to the general immunologic system.
- Gene Name:
- IGHA2
- Uniprot ID:
- P01877
- Molecular Weight:
- 36526.005 Da
References
- Bencko V, Wagner V, Wagnerova M, Reichrtova E: Immuno-biochemical findings in groups of individuals occupationally and non-occupationally exposed to emissions containing nickel and cobalt. J Hyg Epidemiol Microbiol Immunol. 1983;27(4):387-94. [6663071 ]
- General Function:
- Immunoglobulin receptor binding
- Specific Function:
- Not Available
- Gene Name:
- IGHE
- Uniprot ID:
- P01854
- Molecular Weight:
- 47018.665 Da
References
- Shirakawa T, Kusaka Y, Fujimura N, Goto S, Morimoto K: The existence of specific antibodies to cobalt in hard metal asthma. Clin Allergy. 1988 Sep;18(5):451-60. [3233723 ]
- General Function:
- Zinc ion binding
- Specific Function:
- Involved in the base excision repair (BER) pathway, by catalyzing the poly(ADP-ribosyl)ation of a limited number of acceptor proteins involved in chromatin architecture and in DNA metabolism. This modification follows DNA damages and appears as an obligatory step in a detection/signaling pathway leading to the reparation of DNA strand breaks. Mediates the poly(ADP-ribosyl)ation of APLF and CHFR. Positively regulates the transcription of MTUS1 and negatively regulates the transcription of MTUS2/TIP150. With EEF1A1 and TXK, forms a complex that acts as a T-helper 1 (Th1) cell-specific transcription factor and binds the promoter of IFN-gamma to directly regulate its transcription, and is thus involved importantly in Th1 cytokine production. Required for PARP9 and DTX3L recruitment to DNA damage sites. PARP1-dependent PARP9-DTX3L-mediated ubiquitination promotes the rapid and specific recruitment of 53BP1/TP53BP1, UIMC1/RAP80, and BRCA1 to DNA damage sites.
- Gene Name:
- PARP1
- Uniprot ID:
- P09874
- Molecular Weight:
- 113082.945 Da
References
- Hartwig A, Asmuss M, Ehleben I, Herzer U, Kostelac D, Pelzer A, Schwerdtle T, Burkle A: Interference by toxic metal ions with DNA repair processes and cell cycle control: molecular mechanisms. Environ Health Perspect. 2002 Oct;110 Suppl 5:797-9. [12426134 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- Voltage-sensitive calcium channels (VSCC) mediate the entry of calcium ions into excitable cells and are also involved in a variety of calcium-dependent processes, including muscle contraction, hormone or neurotransmitter release, gene expression, cell motility, cell division and cell death. The isoform alpha-1C gives rise to L-type calcium currents. Long-lasting (L-type) calcium channels belong to the 'high-voltage activated' (HVA) group. They are blocked by dihydropyridines (DHP), phenylalkylamines, benzothiazepines, and by omega-agatoxin-IIIA (omega-Aga-IIIA). They are however insensitive to omega-conotoxin-GVIA (omega-CTx-GVIA) and omega-agatoxin-IVA (omega-Aga-IVA). Calcium channels containing the alpha-1C subunit play an important role in excitation-contraction coupling in the heart. The various isoforms display marked differences in the sensitivity to DHP compounds. Binding of calmodulin or CABP1 at the same regulatory sites results in an opposit effects on the channel function.
- Gene Name:
- CACNA1C
- Uniprot ID:
- Q13936
- Molecular Weight:
- 248974.1 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity involved sa node cell action potential
- Specific Function:
- Voltage-sensitive calcium channels (VSCC) mediate the entry of calcium ions into excitable cells and are also involved in a variety of calcium-dependent processes, including muscle contraction, hormone or neurotransmitter release, gene expression, cell motility, cell division and cell death. The isoform alpha-1D gives rise to L-type calcium currents. Long-lasting (L-type) calcium channels belong to the 'high-voltage activated' (HVA) group. They are blocked by dihydropyridines (DHP), phenylalkylamines, benzothiazepines, and by omega-agatoxin-IIIA (omega-Aga-IIIA). They are however insensitive to omega-conotoxin-GVIA (omega-CTx-GVIA) and omega-agatoxin-IVA (omega-Aga-IVA).
- Gene Name:
- CACNA1D
- Uniprot ID:
- Q01668
- Molecular Weight:
- 245138.75 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- Voltage-sensitive calcium channels (VSCC) mediate the entry of calcium ions into excitable cells and are also involved in a variety of calcium-dependent processes, including muscle contraction, hormone or neurotransmitter release, gene expression, cell motility, cell division and cell death. The isoform alpha-1F gives rise to L-type calcium currents. Long-lasting (L-type) calcium channels belong to the 'high-voltage activated' (HVA) group. They are blocked by dihydropyridines (DHP), phenylalkylamines, benzothiazepines, and by omega-agatoxin-IIIA (omega-Aga-IIIA). They are however insensitive to omega-conotoxin-GVIA (omega-CTx-GVIA) and omega-agatoxin-IVA (omega-Aga-IVA).
- Gene Name:
- CACNA1F
- Uniprot ID:
- O60840
- Molecular Weight:
- 220675.9 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- Voltage-sensitive calcium channels (VSCC) mediate the entry of calcium ions into excitable cells and are also involved in a variety of calcium-dependent processes, including muscle contraction, hormone or neurotransmitter release, gene expression, cell motility, cell division and cell death. The isoform alpha-1S gives rise to L-type calcium currents. Long-lasting (L-type) calcium channels belong to the 'high-voltage activated' (HVA) group. They are blocked by dihydropyridines (DHP), phenylalkylamines, benzothiazepines, and by omega-agatoxin-IIIA (omega-Aga-IIIA). They are however insensitive to omega-conotoxin-GVIA (omega-CTx-GVIA) and omega-agatoxin-IVA (omega-Aga-IVA). Calcium channels containing the alpha-1S subunit play an important role in excitation-contraction coupling in skeletal muscle.
- Gene Name:
- CACNA1S
- Uniprot ID:
- Q13698
- Molecular Weight:
- 212348.1 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- The beta subunit of voltage-dependent calcium channels contributes to the function of the calcium channel by increasing peak calcium current, shifting the voltage dependencies of activation and inactivation, modulating G protein inhibition and controlling the alpha-1 subunit membrane targeting.
- Gene Name:
- CACNB1
- Uniprot ID:
- Q02641
- Molecular Weight:
- 65712.995 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- The beta subunit of voltage-dependent calcium channels contributes to the function of the calcium channel by increasing peak calcium current, shifting the voltage dependencies of activation and inactivation, modulating G protein inhibition and controlling the alpha-1 subunit membrane targeting.
- Gene Name:
- CACNB2
- Uniprot ID:
- Q08289
- Molecular Weight:
- 73579.925 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- The beta subunit of voltage-dependent calcium channels contributes to the function of the calcium channel by increasing peak calcium current, shifting the voltage dependencies of activation and inactivation, modulating G protein inhibition and controlling the alpha-1 subunit membrane targeting.
- Gene Name:
- CACNB3
- Uniprot ID:
- P54284
- Molecular Weight:
- 54531.425 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- The beta subunit of voltage-dependent calcium channels contributes to the function of the calcium channel by increasing peak calcium current, shifting the voltage dependencies of activation and inactivation, modulating G protein inhibition and controlling the alpha-1 subunit membrane targeting.
- Gene Name:
- CACNB4
- Uniprot ID:
- O00305
- Molecular Weight:
- 58168.625 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- Voltage-sensitive calcium channels (VSCC) mediate the entry of calcium ions into excitable cells and are also involved in a variety of calcium-dependent processes, including muscle contraction, hormone or neurotransmitter release, gene expression, cell motility, cell division and cell death. The isoform alpha-1B gives rise to N-type calcium currents. N-type calcium channels belong to the 'high-voltage activated' (HVA) group and are blocked by omega-conotoxin-GVIA (omega-CTx-GVIA) and by omega-agatoxin-IIIA (omega-Aga-IIIA). They are however insensitive to dihydropyridines (DHP), and omega-agatoxin-IVA (omega-Aga-IVA). Calcium channels containing alpha-1B subunit may play a role in directed migration of immature neurons.
- Gene Name:
- CACNA1B
- Uniprot ID:
- Q00975
- Molecular Weight:
- 262493.84 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- Voltage-sensitive calcium channels (VSCC) mediate the entry of calcium ions into excitable cells and are also involved in a variety of calcium-dependent processes, including muscle contraction, hormone or neurotransmitter release, gene expression, cell motility, cell division and cell death. The isoform alpha-1A gives rise to P and/or Q-type calcium currents. P/Q-type calcium channels belong to the 'high-voltage activated' (HVA) group and are blocked by the funnel toxin (Ftx) and by the omega-agatoxin-IVA (omega-Aga-IVA). They are however insensitive to dihydropyridines (DHP), and omega-conotoxin-GVIA (omega-CTx-GVIA).
- Gene Name:
- CACNA1A
- Uniprot ID:
- O00555
- Molecular Weight:
- 282362.39 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- Voltage-sensitive calcium channels (VSCC) mediate the entry of calcium ions into excitable cells and are also involved in a variety of calcium-dependent processes, including muscle contraction, hormone or neurotransmitter release, gene expression, cell motility, cell division and cell death. The isoform alpha-1E gives rise to R-type calcium currents. R-type calcium channels belong to the 'high-voltage activated' (HVA) group and are blocked by nickel, and partially by omega-agatoxin-IIIA (omega-Aga-IIIA). They are however insensitive to dihydropyridines (DHP), omega-conotoxin-GVIA (omega-CTx-GVIA), and omega-agatoxin-IVA (omega-Aga-IVA). Calcium channels containing alpha-1E subunit could be involved in the modulation of firing patterns of neurons which is important for information processing.
- Gene Name:
- CACNA1E
- Uniprot ID:
- Q15878
- Molecular Weight:
- 261729.05 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- This protein is a subunit of the dihydropyridine (DHP) sensitive calcium channel. Plays a role in excitation-contraction coupling. The skeletal muscle DHP-sensitive Ca(2+) channel may function only as a multiple subunit complex.
- Gene Name:
- CACNG1
- Uniprot ID:
- Q06432
- Molecular Weight:
- 25028.105 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- Regulates the trafficking and gating properties of AMPA-selective glutamate receptors (AMPARs). Promotes their targeting to the cell membrane and synapses and modulates their gating properties by slowing their rates of activation, deactivation and desensitization. Does not show subunit-specific AMPA receptor regulation and regulates all AMPAR subunits. Thought to stabilize the calcium channel in an inactivated (closed) state.
- Gene Name:
- CACNG2
- Uniprot ID:
- Q9Y698
- Molecular Weight:
- 35965.44 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- Regulates the trafficking and gating properties of AMPA-selective glutamate receptors (AMPARs). Promotes their targeting to the cell membrane and synapses and modulates their gating properties by slowing their rates of activation, deactivation and desensitization. Does not show subunit-specific AMPA receptor regulation and regulates all AMPAR subunits. Thought to stabilize the calcium channel in an inactivated (closed) state (By similarity).
- Gene Name:
- CACNG3
- Uniprot ID:
- O60359
- Molecular Weight:
- 35548.14 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- Regulates the trafficking and gating properties of AMPA-selective glutamate receptors (AMPARs). Promotes their targeting to the cell membrane and synapses and modulates their gating properties by slowing their rates of activation, deactivation and desensitization and by mediating their resensitization. Does not show subunit-specific AMPA receptor regulation and regulates all AMPAR subunits. Thought to stabilize the calcium channel in an inactivated (closed) state.
- Gene Name:
- CACNG4
- Uniprot ID:
- Q9UBN1
- Molecular Weight:
- 36578.39 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- Regulates the gating properties of AMPA-selective glutamate receptors (AMPARs). Modulates their gating properties by accelerating their rates of activation, deactivation and desensitization. Displays subunit-specific AMPA receptor regulation. Shows specificity for GRIA1, GRIA4 and the long isoform of GRIA2. Thought to stabilize the calcium channel in an inactivated (closed) state (By similarity).
- Gene Name:
- CACNG5
- Uniprot ID:
- Q9UF02
- Molecular Weight:
- 30902.44 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- Thought to stabilize the calcium channel in an inactivated (closed) state.
- Gene Name:
- CACNG6
- Uniprot ID:
- Q9BXT2
- Molecular Weight:
- 28128.745 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- Regulates the trafficking and gating properties of AMPA-selective glutamate receptors (AMPARs). Promotes their targeting to the cell membrane and synapses and modulates their gating properties by slowing their rates of activation, deactivation and desensitization and by mediating their resensitization. Displays subunit-specific AMPA receptor regulation. Shows specificity only for GRIA1 and GRIA2. Thought to stabilize the calcium channel in an inactivated (closed) state.
- Gene Name:
- CACNG7
- Uniprot ID:
- P62955
- Molecular Weight:
- 31002.29 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- Regulates the trafficking and gating properties of AMPA-selective glutamate receptors (AMPARs). Promotes their targeting to the cell membrane and synapses and modulates their gating properties by slowing their rates of activation, deactivation and desensitization and by mediating their resensitization. Does not show subunit-specific AMPA receptor regulation and regulates all AMPAR subunits. Thought to stabilize the calcium channel in an inactivated (closed) state.
- Gene Name:
- CACNG8
- Uniprot ID:
- Q8WXS5
- Molecular Weight:
- 43312.44 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated ion channel activity
- Specific Function:
- Thought to stabilize the calcium channel in an inactivated (closed) state. Modulates calcium current when coexpressed with CACNA1G (By similarity).
- Gene Name:
- TMEM37
- Uniprot ID:
- Q8WXS4
- Molecular Weight:
- 20931.565 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- The alpha-2/delta subunit of voltage-dependent calcium channels regulates calcium current density and activation/inactivation kinetics of the calcium channel. Plays an important role in excitation-contraction coupling (By similarity).
- Gene Name:
- CACNA2D1
- Uniprot ID:
- P54289
- Molecular Weight:
- 124566.93 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- The alpha-2/delta subunit of voltage-dependent calcium channels regulates calcium current density and activation/inactivation kinetics of the calcium channel. Acts as a regulatory subunit for P/Q-type calcium channel (CACNA1A), N-type (CACNA1B), L-type (CACNA1C OR CACNA1D) and possibly T-type (CACNA1G). Overexpression induces apoptosis.
- Gene Name:
- CACNA2D2
- Uniprot ID:
- Q9NY47
- Molecular Weight:
- 129816.095 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated ion channel activity
- Specific Function:
- The alpha-2/delta subunit of voltage-dependent calcium channels regulates calcium current density and activation/inactivation kinetics of the calcium channel. Acts as a regulatory subunit for P/Q-type calcium channel (CACNA1A), N-type (CACNA1B), L-type (CACNA1C OR CACNA1D) but not T-type (CACNA1G) (By similarity).
- Gene Name:
- CACNA2D3
- Uniprot ID:
- Q8IZS8
- Molecular Weight:
- 123010.22 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- The alpha-2/delta subunit of voltage-dependent calcium channels regulates calcium current density and activation/inactivation kinetics of the calcium channel.
- Gene Name:
- CACNA2D4
- Uniprot ID:
- Q7Z3S7
- Molecular Weight:
- 127936.93 Da
References
- Castelli L, Tanzi F, Taglietti V, Magistretti J: Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol. 2003 Oct 1;195(3):121-36. [14724759 ]