| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-05-25 21:11:21 UTC |
|---|
| Update Date | 2014-12-24 20:22:49 UTC |
|---|
| Accession Number | T3D0782 |
|---|
| Identification |
|---|
| Common Name | 1,3-Dinitrobenzene |
|---|
| Class | Small Molecule |
|---|
| Description | 1,3-Dinitrobenzene (1,3-DNB) is a synthetic substance that is used in explosives. Nitrite is a toxic compound known to cause methemoglobinemia. (8, 7) |
|---|
| Compound Type | - Amine
- Aromatic Hydrocarbon
- Explosive Agent
- Industrial/Workplace Toxin
- Nitrate
- Organic Compound
- Synthetic Compound
|
|---|
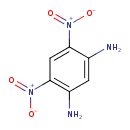
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1,3-Dinitro-benzen | | 1,3-Dinitrobenzol | | 2,4-Dinitrobenzene | | 4,6-dinitro-1,3-benzenediamine | | Binitrobenzene | | m-Dinitro-benzene | | m-Dinitrobenzene | | NIN |
|
|---|
| Chemical Formula | C6H6N4O4 |
|---|
| Average Molecular Mass | 198.136 g/mol |
|---|
| Monoisotopic Mass | 198.039 g/mol |
|---|
| CAS Registry Number | 99-65-0 |
|---|
| IUPAC Name | 4,6-dinitrobenzene-1,3-diamine |
|---|
| Traditional Name | 4,6-dinitrobenzene-1,3-diamine |
|---|
| SMILES | NC1=CC(N)=C(C=C1[N+]([O-])=O)[N+]([O-])=O |
|---|
| InChI Identifier | InChI=1S/C6H6N4O4/c7-3-1-4(8)6(10(13)14)2-5(3)9(11)12/h1-2H,7-8H2 |
|---|
| InChI Key | InChIKey=DFBUFGZWPXQRJV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dinitroanilines. These are organic compounds containing an aniline moiety, which is substituted at 2 positions by a nitro group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Aniline and substituted anilines |
|---|
| Direct Parent | Dinitroanilines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dinitroaniline
- Nitrobenzene
- Nitroaromatic compound
- C-nitro compound
- Organic nitro compound
- Organic oxoazanium
- Allyl-type 1,3-dipolar organic compound
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Organic nitrogen compound
- Organic zwitterion
- Primary amine
- Organonitrogen compound
- Amine
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | Yellow solid. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 90°C | | Boiling Point | Not Available | | Solubility | 0.533 mg/mL at 25°C [SPANGGORD,RJ et al. (1980)] | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-d0da97c87af62b76ad50 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006y-0900000000-7cd28fa04d83f5ef3930 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ry-0900000000-e04318ea9f6067a3305e | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-47416b6028f6eee611f1 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0900000000-58a12a85abaa30bee99c | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000e-1900000000-9432f54780cfbfa8ffbf | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (7) ; inhalation (7) ; dermal (7) |
|---|
| Mechanism of Toxicity | In the red blood cell, 1,3-DNB induces formation of methemoglobin, leading to cyanosis. Reduction of the nitrogroup(s) of 1,3-DNB produces reactive nitroaromatic radical anions which redox cycle to produce other reactive, toxic species such as superoxide anion. Redox cycling of these intermediates probably causes the methemoglobinemia. In the male reproductive system, 1,3-DNB causes disruption of spermatogenesis resulting in hypospermia, poor sperm quality, and infertility. Reduction of 1,3-DNB to reactive species such as nitrosonitrobenzene is believed to damage Sertoli cells. (7) |
|---|
| Metabolism | 1,3-Dinitrobenzene is absorbed via oral, inhalation, and dermal routes and is believed to be able to penetrate the red blood cell membrane. The metabolism of 1,3-DNB includes both oxidative and reductive biotransformations, followed by conjugation. The main metabolites are 3-aminoacetanilide, 4-acetamidophenylsulfate, 1,3-diacetamidobenzene, and 3-nitroaniline-N-glucuronide. The main route of excretion of 1,3-DNB metabolites is the urine. (7) |
|---|
| Toxicity Values | LD50: 59 mg/kg (Oral, Rat) (7)
LD50: 1990 mg/kg (Dermal, Rabbit) (7)
LD50: 10 mg/kg (Intravenous, Dog) (2)
LD50: 28 mg/kg (Intraperitoneal, Rat) (2) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity (not listed by IARC). (5) |
|---|
| Uses/Sources | 1,3-Dinitrobenzene is used in explosives. Waste discharges from army ammunitions plants or other chemical manufacturers are the primary sources for release into the air, water, and soil. (7) |
|---|
| Minimum Risk Level | Acute Oral: 0.008 mg/kg/day (4)
Intermediate Oral: 0.0005 mg/kg/day (4) |
|---|
| Health Effects | Chronic exposure to 1,3-dinitrobenzene can cause a reduction (or loss) in the number of red blood cells (anemia). It may also cause behavioral changes and male reproductive system damage. (7) |
|---|
| Symptoms | Exposure to high concentrations of 1,3-dinitrobenzene can reduce the ability of blood to carry oxygen and can cause your skin to become bluish in color. Other symptoms of 1,3-dinitrobenzene exposure include headache, nausea, and dizziness.(7) |
|---|
| Treatment | Treatment is mainly symptomatic and may include gastric lavage and/or the administration of activated charcoal. Benzodiazepine may be administered if seizures occur. If methemoglobinemia is evident, 1 to 2 mg/kg of 1% methylene blue should be administered via IV. (3) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 291796 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 257460 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | C017906 |
|---|
| Stitch ID | 1,3-Dinitrobenzene |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 6434 |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D0782.pdf |
|---|
| General References | - Keszler A, Piknova B, Schechter AN, Hogg N: The reaction between nitrite and oxyhemoglobin: a mechanistic study. J Biol Chem. 2008 Apr 11;283(15):9615-22. doi: 10.1074/jbc.M705630200. Epub 2008 Jan 17. [18203719 ]
- Lewis RJ (1996). Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold.
- Rumack BH (2009). POISINDEX(R) Information System. Englewood, CO: Micromedex, Inc. CCIS Volume 141, edition expires Aug, 2009.
- ATSDR - Agency for Toxic Substances and Disease Registry (2001). Minimal Risk Levels (MRLs) for Hazardous Substances. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2008). Toxicological profile for diazinon. U.S. Public Health Service in collaboration with U.S. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (1995). Toxicological profile for 1,3-dinitrobenzene and 1,3,5-trinitrobenzene (1,3-DNB and 1,3,5-TNB). U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2007). Case Studies in Environmental Medicine. Nitrate/Nitrite Toxicity. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Methemoglobinemia. Last Updated 22 July 2009. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|