| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-06-15 17:20:33 UTC |
|---|
| Update Date | 2014-12-24 20:22:54 UTC |
|---|
| Accession Number | T3D0870 |
|---|
| Identification |
|---|
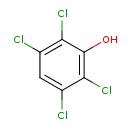
| Common Name | 2,3,5,6-Tetrachlorophenol |
|---|
| Class | Small Molecule |
|---|
| Description | 2,3,5,6-Tetrachlorophenol is a biodegradation product of polychlorinated benzene and polychlorinated biphenols. |
|---|
| Compound Type | - Aromatic Hydrocarbon
- Food Toxin
- Lachrymator
- Organic Compound
- Organochloride
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 2,3,5, 6-Tetrachlorophenol | | 2,3,5,6-Tetrachloro phenol | | 2,3,5,6-Tetrachlorophenate | | 2,3,5,6-Tetrachlorophenol solution | | WLN: QR bg CG eg FG |
|
|---|
| Chemical Formula | C6H2Cl4O |
|---|
| Average Molecular Mass | 231.891 g/mol |
|---|
| Monoisotopic Mass | 229.886 g/mol |
|---|
| CAS Registry Number | 935-95-5 |
|---|
| IUPAC Name | 2,3,5,6-tetrachlorophenol |
|---|
| Traditional Name | 2,3,5,6-tetrachlorophenol |
|---|
| SMILES | OC1=C(Cl)C(Cl)=CC(Cl)=C1Cl |
|---|
| InChI Identifier | InChI=1S/C6H2Cl4O/c7-2-1-3(8)5(10)6(11)4(2)9/h1,11H |
|---|
| InChI Key | InChIKey=KEWNKZNZRIAIAK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as o-chlorophenols. These are chlorophenols carrying a iodine at the C2 position of the benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | Halophenols |
|---|
| Direct Parent | O-chlorophenols |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-chlorophenol
- 3-chlorophenol
- 1-hydroxy-4-unsubstituted benzenoid
- Halobenzene
- Chlorobenzene
- Monocyclic benzene moiety
- Aryl halide
- Aryl chloride
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organochloride
- Organohalogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 115°C | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2022-08-08 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0090000000-48c0be405602c36c1526 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0090000000-e538b04aa45113cfaff1 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002f-0970000000-f2a72c45f0df9c0d74cf | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0090000000-08217b277413993527c9 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0090000000-4c84de79bc4bbc2eca0a | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000x-0960000000-450f19d37b47584e56dd | 2016-06-03 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-001i-4690000000-d32a2509948e34635636 | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100.53 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Inhalation (5) ; oral (5) ; dermal (5) ; eye contact (5) |
|---|
| Mechanism of Toxicity | 2,3,5,6-tetrachlorophenol acts at the sites of adenosine triphosphate production and decreases or blocks it without blocking the electron transport chain. Thus the poison uncouples phosphorylation from oxidation. Free energy from the electron transport chain then converts to more body heat. As body temp rises, heat-dissipating mechanisms are overcome and metabolism is speeded. More adenosine diphosphate and other substrates accumulate, and these substrates stimulate the electron transport chain further. The electron transport chain responds by using up more and more available oxygen (increasing oxygen demand) in an effort to produce adenosine triphosphate, but much of the free energy generated is liberated as still more body heat. Oxygen demand quickly overcomes oxygen supply, and energy reserves become depleted (1). |
|---|
| Metabolism | 2,3,5,6-Tetrachlorophenol is rapidly absorbed and excreted following occupational exposure, which involves both the inhalation and dermal routes. Sulfation and glucuronidation are the main metabolic pathways of 2,3,5,6-tetrachlorophenol. Studies suggest rapid absorption after dermal application. 2,3,5,6-Tetrachlorophenol is excreted as tetrachloro-p-hydroquinone, and partly unchanged (5). |
|---|
| Toxicity Values | LD50: 89 mg/kg (Oral, Mouse) (5)
LD50: >2000 mg/kg (Dermal, Rabbit) (5) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not directly listed by IARC, but related polychlorophenols are discussed, and combined exposures to polychlorophenols or to their sodium salts are classified as possibly carcinogenic to humans (Group 2B). (4) |
|---|
| Uses/Sources | Breathing contamiated air; drinking contaminated water; eating contaminating food; skin/eye contact (particularly while treating wood) (5). |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Dermal exposure can cause corrosive skin damage (5). |
|---|
| Symptoms | Dust has been found irritating to the nose and throat. Skin or eye contact can cause irritation of the exposed surface. Clinical signs preceding death for all tetrachlorophenol isomers included initial hyperactivity followed by hypoactivity, neuromuscular weakness, and convulsions. Headache can also result from exposure to 2,3,5,6-tetrachlorophenol (5). |
|---|
| Treatment | In case of oral exposure, dilution may enhance absorption of phenol, and should be avoided; charcoal may be administered. If methemoglobinemia occurs, administer 1 to 2 mg/kg of 1% methylene blue slowly IV in symptomatic patients. Additional doses may be required. if hypotensin occurs, infuse 10 to 20 mL/kg isotonic fluid. If hypotension persists, administer dopamine or norepinephrine. Following inhalation exposure, move patient to fresh air. Monitor for respiratory distress, and if cough or difficulty breathing develops, evaluate for respiratory tract irritation, bronchitis, or pneumonitis. Administer oxygen and assist ventilation as required. Treat bronchospasm with inhaled beta2 agonist and oral or parenteral corticosteroids. Irrigate exposed eyes with copious amounts of room temperature water for at least 15 minutes following eye exposure. Following dermal exposure, remove phenol with undiluted polyethylene glycol 300 to 400 or isopropyl alcohol prior to washing, if readily available. Wash exposed areas twice or for at least 10 minutes with large quantities of soapy water. Water alone may be harmful. (2) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 13636 |
|---|
| ChEMBL ID | CHEMBL1606550 |
|---|
| ChemSpider ID | 13047 |
|---|
| KEGG ID | C15505 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 52048 |
|---|
| BioCyc ID | DIHYDRO-DIOH-BENZOATE |
|---|
| CTD ID | C039673 |
|---|
| Stitch ID | 2,3,5,6-Tetrachlorophenol |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - Huq MD, Tsai NP, Gupta P, Wei LN: Regulation of retinal dehydrogenases and retinoic acid synthesis by cholesterol metabolites. EMBO J. 2006 Jul 12;25(13):3203-13. Epub 2006 Jun 8. [16763553 ]
- Rumack BH (2009). POISINDEX(R) Information System. Englewood, CO: Micromedex, Inc. CCIS Volume 141, edition expires Aug, 2009.
- Booth, NH, McDonald LE (eds) (1982). Veterinary Pharmacology and Therapeutics. 5th ed. Ames, Iowa: Iowa State University Press.
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (1999). Toxicological profile for chlorophenols. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|