| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-06-19 21:58:26 UTC |
|---|
| Update Date | 2014-12-24 20:23:23 UTC |
|---|
| Accession Number | T3D1206 |
|---|
| Identification |
|---|
| Common Name | Copper(II) azide |
|---|
| Class | Small Molecule |
|---|
| Description | Copper(II) azide is a chemical compound of copper. It is very explosive and can be used in pyrotechnics. Copper is a chemical element with the symbol Cu and atomic number 29. Copper is an essential elements in plants and animals as it is required for the normal functioning of more than 30 enzymes. It occurs naturally throughout the environment in rocks, soil, water, and air. (6, 7, 10) |
|---|
| Compound Type | - Copper Compound
- Industrial/Workplace Toxin
- Inorganic Compound
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | |
|---|
| Chemical Formula | CuN6 |
|---|
| Average Molecular Mass | 147.586 g/mol |
|---|
| Monoisotopic Mass | 146.948 g/mol |
|---|
| CAS Registry Number | 14215-30-6 |
|---|
| IUPAC Name | copper(2+) ion bis(2λ⁵-triaz-1-en-2-yn-1-ide) |
|---|
| Traditional Name | copper(2+) ion bis(azide) |
|---|
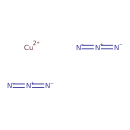
| SMILES | [Cu++].[N-]=[N+]=[N-].[N-]=[N+]=[N-] |
|---|
| InChI Identifier | InChI=1S/Cu.2N3/c;2*1-3-2/q+2;2*-1 |
|---|
| InChI Key | InChIKey=SXHYOTRZGGGMEV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as transition metal nitrides. These are inorganic compounds of nitrogen where nitrogen has a formal oxidation state of -3, and the heaviest metal atom is a transition metal. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Mixed metal/non-metal compounds |
|---|
| Class | Transition metal organides |
|---|
| Sub Class | Transition metal nitrides |
|---|
| Direct Parent | Transition metal nitrides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Transition metal nitride
- Inorganic copper salt
- Inorganic nitride
- Inorganic salt
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | Dark green crystalline solid. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | (explodes) | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-9f72bed8c761e83f4a74 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0900000000-9f72bed8c761e83f4a74 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-0900000000-9f72bed8c761e83f4a74 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-19f9b4a759dda704a096 | 2016-08-04 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0900000000-19f9b4a759dda704a096 | 2016-08-04 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-0900000000-19f9b4a759dda704a096 | 2016-08-04 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (6) ; inhalation (6) ; dermal (6) |
|---|
| Mechanism of Toxicity | Excess copper is sequestered within hepatocyte lysosomes, where it is complexed with metallothionein. Copper hepatotoxicity is believed to occur when the lysosomes become saturated and copper accumulates in the nucleus, causing nuclear damage. This damage is possibly a result of oxidative damage, including lipid peroxidation. Copper inhibits the sulfhydryl group enzymes such as glucose-6-phosphate 1-dehydrogenase, glutathione reductase, and paraoxonases, which protect the cell from free oxygen radicals. It also influences gene expression and is a co-factor for oxidative enzymes such as cytochrome C oxidase and lysyl oxidase. In addition, the oxidative stress induced by copper is thought to activate acid sphingomyelinase, which lead to the production of ceramide, an apoptotic signal, as well as cause hemolytic anemia. Copper-induced emesis results from stimulation of the vagus nerve. (6, 4, 1, 9) |
|---|
| Metabolism | Copper is mainly absorbed through the gastrointestinal tract, but it can also be inhalated and absorbed dermally. It passes through the basolateral membrane, possibly via regulatory copper transporters, and is transported to the liver and kidney bound to serum albumin. The liver is the critical organ for copper homoeostasis. In the liver and other tissues, copper is stored bound to metallothionein, amino acids, and in association with copper-dependent enzymes, then partitioned for excretion through the bile or incorporation into intra- and extracellular proteins. The transport of copper to the peripheral tissues is accomplished through the plasma attached to serum albumin, ceruloplasmin or low-molecular-weight complexes. Copper may induce the production of metallothionein and ceruloplasmin. The membrane-bound copper transporting adenosine triphosphatase (Cu-ATPase) transports copper ions into and out of cells. Physiologically normal levels of copper in the body are held constant by alterations in the rate and amount of copper absorption, compartmental distribution, and excretion. (6, 8) |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | 10 to 20 grams for an adult human (copper salts). (3) |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Copper(II) azide is very explosive and can be used in pyrotechnics. (10) |
|---|
| Minimum Risk Level | Acute Oral: 0.01 mg/kg/day (5)
Intermediate Oral: 0.01 mg/kg/day (5) |
|---|
| Health Effects | People must absorb small amounts of copper every day because copper is essential for good health, however, high levels of copper can be harmful. Very-high doses of copper can cause damage to your liver and kidneys, and can even cause death. Copper may induce allergic responses in sensitive individuals. (7, 8) |
|---|
| Symptoms | Breathing high levels of copper can cause irritation of the nose and throat. Ingesting high levels of copper can cause nausea, vomiting, diarrhea, headache, dizziness, and respiratory difficulty. (7, 8) |
|---|
| Treatment | EYES: irrigate opened eyes for several minutes under running water. INGESTION: do not induce vomiting. Rinse mouth with water (never give anything by mouth to an unconscious person). Seek immediate medical advice. SKIN: should be treated immediately by rinsing the affected parts in cold running water for at least 15 minutes, followed by thorough washing with soap and water. If necessary, the person should shower and change contaminated clothing and shoes, and then must seek medical attention. INHALATION: supply fresh air. If required provide artificial respiration. |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 5743369 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | Not Available |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Copper(II) azide |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - Brewer GJ: A brand new mechanism for copper toxicity. J Hepatol. 2007 Oct;47(4):621-2. Epub 2007 Jul 23. [17697726 ]
- Bardsley PA, Howard P, DeBacker W, Vermeire P, Mairesse M, Ledent C, Radermecker M, Bury T, Ansquer J: Two years treatment with almitrine bismesylate in patients with hypoxic chronic obstructive airways disease. Eur Respir J. 1991 Mar;4(3):308-10. [1907566 ]
- Baselt RC (2000). Disposition of Toxic Drugs and Chemicals in Man, 5th ed. Foster City, CA: Chemical Toxicology Institute.
- Baxter PJ, Adams PH, & Aw TC (2000). Hunter's Diseases of Occupations. 9th ed. New York, NY: Oxford University Press Inc.
- ATSDR - Agency for Toxic Substances and Disease Registry (2001). Minimal Risk Levels (MRLs) for Hazardous Substances. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Copper. Last Updated 29 May 2009. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2004). Toxicological profile for copper. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- International Programme on Chemical Safety (IPCS) INCHEM (1998). Environmental Health Criteria for Copper. [Link]
- US Environmental Protection Agency (2008). Drinking Water Health Advisory for 2,4-Dinitrotoluene and 2,6-Dinitrotoluene. [Link]
- Wikipedia. Copper(II) azide. Last Updated 17 May 2009. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|