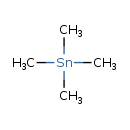| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-06-19 21:58:31 UTC |
|---|
| Update Date | 2014-12-24 20:23:31 UTC |
|---|
| Accession Number | T3D1262 |
|---|
| Identification |
|---|
| Common Name | Tetramethyltin |
|---|
| Class | Small Molecule |
|---|
| Description | Tetramethyltin is an organotin compound. It is used in organic synthesis for transition-metal mediated conversion of acid chlorides to methyl ketones and aryl halides to aryl methyl ketones. Tin is a chemical element with the symbol Sn and atomic number 50. It is a natural component of the earth's crust and is obtained chiefly from the mineral cassiterite, where it occurs as tin dioxide. (3, 5, 6) |
|---|
| Compound Type | - Industrial/Workplace Toxin
- Organic Compound
- Organometallic
- Synthetic Compound
- Tin Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (CH3)4sn | | SnMe4 | | Tetramethyl tin | | Tetramethylstannane | | Tin tetramethyl | | [SnMe4] |
|
|---|
| Chemical Formula | C4H12Sn |
|---|
| Average Molecular Mass | 178.850 g/mol |
|---|
| Monoisotopic Mass | 179.996 g/mol |
|---|
| CAS Registry Number | 594-27-4 |
|---|
| IUPAC Name | tetramethylstannane |
|---|
| Traditional Name | tetramethyltin |
|---|
| SMILES | C[Sn](C)(C)C |
|---|
| InChI Identifier | InChI=1S/4CH3.Sn/h4*1H3; |
|---|
| InChI Key | InChIKey=VXKWYPOMXBVZSJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetraalkyltins. These are tetraorganotin compounds where the tin atom is linked to exactly four alkyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organometallic compounds |
|---|
| Class | Organo-post-transition metal compounds |
|---|
| Sub Class | Organotin compounds |
|---|
| Direct Parent | Tetraalkyltins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetraalkyltin
- Hydrocarbon derivative
- Organic salt
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | -54.8°C | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-b726ba190e5cb769bfa3 | 2019-02-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0900000000-99793dc219220851fc35 | 2019-02-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-1900000000-772ca63625d491b111e4 | 2019-02-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01t9-0900000000-40b735d92de2cfe78db8 | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-1900000000-ce8c8b6e46954728304a | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0900000000-7f1823ae098cf3d5af64 | 2019-02-23 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-03xr-0900000000-25e387851425fac2b1ef | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 90 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (4) ; inhalation (4) ; dermal (4) |
|---|
| Mechanism of Toxicity | Organotin compounds produce neurotoxic and immunotoxic effects. Organotins may directly activate glial cells contributing to neuronal cell degeneration by local release of pro-inflammatory cytokines, tumor necrosis factor-_, and/or interleukins. They may also induce apoptosis by direct action on neuronal cells. Organotin compounds stimulate the neuronal release of and/or decrease of neuronal cell uptake of neurotransmitters in brain tissue, including aspartate, GABA, glutamate, norepinephrine, and serotonin. This may be either a contributing factor to or result of the neuronal cell loss. The immunotoxic effects of organotins are characterized by thymic atrophy caused by the suppression of proliferation of immature thymocytes and apoptosis of mature thymocytes. Organotin compounds are believed to exert these effects by suppressing DNA and protein synthesis, inducing the expression of genes involved in apoptosis (such as nur77), and disrupting the regulation of intracellular calcium levels, giving rise to the uncontrolled production of reactive oxygen species, release of cytochrome c to the cytosol, and the proteolytic and nucleolytic cascade of apoptosis. The suppression of proliferation of immature thymocytes further results in the suppression of T-cell-mediated immune responses. Organotins are also endocrine disruptors and are believed to contribute to obesity by inappropriate receptor activation, leading to adipocyte differentiation. Inorganic tin triggers eryptosis, contributing to tin-induced anemia. (4, 1, 2) |
|---|
| Metabolism | Though tin metal is very poorly absorbed, tin compounds may be absorbed via oral, inhalation, or dermal routes, with organotin compounds being much more readily absorbed than inorganic tin compounds. Tin may enter the bloodstream and bind to hemoglobin, where it is distributed and accumulates mainly in the kidney, liver, lung, and bone. Organotin compounds may undergo dealkylation, hydroxylation, dearylation, and oxidation catalyzed by cytochrome P-450 enzymes in the liver. The alkyl products of dealkylation are conjugated with glutathione and further metabolized to mercapturic acid derivatives. Tin and its metabolites are excreted mainly in the urine and feces. (4) |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Tetramethyltin is used in organic synthesis for transition-metal mediated conversion of acid chlorides to methyl ketones and aryl halides to aryl methyl ketones. (6) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Breathing or swallowing, or skin contact with organotins, can interfere with the way the brain and nervous system work, causing death in severe cases. Organic tin compounds may also damage the immune and reproductive system. (3, 4) |
|---|
| Symptoms | Inorganic or organic tin compounds placed on the skin or in the eyes can produce skin and eye irritation. (4) |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 11661 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 11171 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 30420 |
|---|
| BioCyc ID | CPD-10343 |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Tetramethyltin |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 10067 |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D1262.pdf |
|---|
| General References | - Nguyen TT, Foller M, Lang F: Tin triggers suicidal death of erythrocytes. J Appl Toxicol. 2009 Jan;29(1):79-83. doi: 10.1002/jat.1390. [18937211 ]
- Grun F, Blumberg B: Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006 Jun;147(6 Suppl):S50-5. Epub 2006 May 11. [16690801 ]
- Wikipedia. Tributyltin. Last Updated 31 May 2009. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2005). Toxicological profile for tin. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Tin. Last Updated 28 May 2009. [Link]
- Wikipedia. Tetramethyltin. Last Updated 14 April 2009. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|