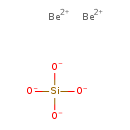
Beryllium silicate (T3D1375)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-06-19 21:58:41 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:23:48 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D1375 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Beryllium silicate | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Beryllium silicate is a chemical compound of beryllium. Beryllium is a lightweight alkaline earth metal with the atomic number 4. It is a relatively rare element found naturally only combined with other elements in minerals. (3) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | Be2O4Si | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 110.108 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 109.981 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 13598-00-0 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | diberyllium(2+) ion silicate | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | diberyllium(2+) ion orthosilicate | |||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | [Be++].[Be++].[O-][Si]([O-])([O-])[O-] | |||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/2Be.O4Si/c;;1-5(2,3)4/q2*+2;-4 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=QZVSYHUREAVHQG-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of inorganic compounds known as alkaline earth metal silicates. These are inorganic compounds in which the largest oxoanion is silicate, and in which the heaviest atom not in an oxoanion is an alkaline earth metal. | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Inorganic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Mixed metal/non-metal compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Alkaline earth metal oxoanionic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Alkaline earth metal silicates | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Alkaline earth metal silicates | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | White powder. | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Inhalation (3) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Once in the body, beryllium acts as a hapten and interacts with human leucocyte antigen (HLA) DP presenting cells in the lungs, becoming physically associated with a major histocompatability (MHC) class II molecule. This MHC class II-beryllium-peptide complex is recognized by the T lymphocyte receptor, triggering CD4+ T lymphocyte activation and proliferation. The resulting inflammatory response is a cell-mediated process orchestrated by cytokines and results in the formation of (usually pulmonary) granulomas. Beryllium's toxicity may be controlled by the iron-storage protein ferritin, which sequesters beryllium by binding it and preventing it from interacting with other enzymes. (4, 1, 2) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Beryllium is absorbed mainly through the lungs, where it enters the bloodstream and is transported throughout the body by binding to prealbumins and _-globulins. Beryllium accumulates in lung tissue and the skeleton. It is excreted mainly in the urine. (4) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | 1, carcinogenic to humans. (6) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Chronic Oral: 0.002 mg/kg/day (5) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Acute inhalation of a high level of beryllium can results in a pneumonia-like condition called acute beryllium disease. Chronic inhalation of beryllium can caused an inflammatory reaction in the respiratory system called chronic beryllium disease. Chronic beryllium disease may result in anorexia and weight loss, as well as right side heart enlargement and heart disease in advanced cases. Chronic exposure can also increase the risk of lung cancer. Skin contact with beryllium results in contact dermatitus. (3, 4) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Chronic beryllium disease causes fatigue, weakness, difficulty breathing, and a persistent dry cough. (3, 4) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Chronic beryllium disease is treated with immunosuppressive medicines, usually of the glucocorticoid class. (3) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 27131 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 25251 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Beryllium silicate | |||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Peptide antigen binding
- Specific Function:
- Binds peptides derived from antigens that access the endocytic route of antigen presenting cells (APC) and presents them on the cell surface for recognition by the CD4 T-cells. The peptide binding cleft accommodates peptides of 10-30 residues. The peptides presented by MHC class II molecules are generated mostly by degradation of proteins that access the endocytic route, where they are processed by lysosomal proteases and other hydrolases. Exogenous antigens that have been endocytosed by the APC are thus readily available for presentation via MHC II molecules, and for this reason this antigen presentation pathway is usually referred to as exogenous. As membrane proteins on their way to degradation in lysosomes as part of their normal turn-over are also contained in the endosomal/lysosomal compartments, exogenous antigens must compete with those derived from endogenous components. Autophagy is also a source of endogenous peptides, autophagosomes constitutively fuse with MHC class II loading compartments. In addition to APCs, other cells of the gastrointestinal tract, such as epithelial cells, express MHC class II molecules and CD74 and act as APCs, which is an unusual trait of the GI tract. To produce a MHC class II molecule that presents an antigen, three MHC class II molecules (heterodimers of an alpha and a beta chain) associate with a CD74 trimer in the ER to form a heterononamer. Soon after the entry of this complex into the endosomal/lysosomal system where antigen processing occurs, CD74 undergoes a sequential degradation by various proteases, including CTSS and CTSL, leaving a small fragment termed CLIP (class-II-associated invariant chain peptide). The removal of CLIP is facilitated by HLA-DM via direct binding to the alpha-beta-CLIP complex so that CLIP is released. HLA-DM stabilizes MHC class II molecules until primary high affinity antigenic peptides are bound. The MHC II molecule bound to a peptide is then transported to the cell membrane surface. In B-cells, the interaction between HLA-DM and MHC class II molecules is regulated by HLA-DO. Primary dendritic cells (DCs) also to express HLA-DO. Lysosomal microenvironment has been implicated in the regulation of antigen loading into MHC II molecules, increased acidification produces increased proteolysis and efficient peptide loading.
- Gene Name:
- HLA-DPA1
- Uniprot ID:
- P20036
- Molecular Weight:
- 29380.345 Da
References
- Amicosante M, Berretta F, Dweik R, Saltini C: Role of high-affinity HLA-DP specific CLIP-derived peptides in beryllium binding to the HLA-DPGlu69 berylliosis-associated molecules and presentation to beryllium-sensitized T cells. Immunology. 2009 Sep;128(1 Suppl):e462-70. doi: 10.1111/j.1365-2567.2008.03000.x. Epub 2008 Dec 23. [19191908 ]
- ATSDR - Agency for Toxic Substances and Disease Registry (2002). Toxicological profile for beryllium. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- General Function:
- Peptide antigen binding
- Specific Function:
- Binds peptides derived from antigens that access the endocytic route of antigen presenting cells (APC) and presents them on the cell surface for recognition by the CD4 T-cells. The peptide binding cleft accommodates peptides of 10-30 residues. The peptides presented by MHC class II molecules are generated mostly by degradation of proteins that access the endocytic route, where they are processed by lysosomal proteases and other hydrolases. Exogenous antigens that have been endocytosed by the APC are thus readily available for presentation via MHC II molecules, and for this reason this antigen presentation pathway is usually referred to as exogenous. As membrane proteins on their way to degradation in lysosomes as part of their normal turn-over are also contained in the endosomal/lysosomal compartments, exogenous antigens must compete with those derived from endogenous components. Autophagy is also a source of endogenous peptides, autophagosomes constitutively fuse with MHC class II loading compartments. In addition to APCs, other cells of the gastrointestinal tract, such as epithelial cells, express MHC class II molecules and CD74 and act as APCs, which is an unusual trait of the GI tract. To produce a MHC class II molecule that presents an antigen, three MHC class II molecules (heterodimers of an alpha and a beta chain) associate with a CD74 trimer in the ER to form a heterononamer. Soon after the entry of this complex into the endosomal/lysosomal system where antigen processing occurs, CD74 undergoes a sequential degradation by various proteases, including CTSS and CTSL, leaving a small fragment termed CLIP (class-II-associated invariant chain peptide). The removal of CLIP is facilitated by HLA-DM via direct binding to the alpha-beta-CLIP complex so that CLIP is released. HLA-DM stabilizes MHC class II molecules until primary high affinity antigenic peptides are bound. The MHC II molecule bound to a peptide is then transported to the cell membrane surface. In B-cells, the interaction between HLA-DM and MHC class II molecules is regulated by HLA-DO. Primary dendritic cells (DCs) also to express HLA-DO. Lysosomal microenvironment has been implicated in the regulation of antigen loading into MHC II molecules, increased acidification produces increased proteolysis and efficient peptide loading.
- Gene Name:
- HLA-DPB1
- Uniprot ID:
- P04440
- Molecular Weight:
- 29159.195 Da
References
- Amicosante M, Berretta F, Dweik R, Saltini C: Role of high-affinity HLA-DP specific CLIP-derived peptides in beryllium binding to the HLA-DPGlu69 berylliosis-associated molecules and presentation to beryllium-sensitized T cells. Immunology. 2009 Sep;128(1 Suppl):e462-70. doi: 10.1111/j.1365-2567.2008.03000.x. Epub 2008 Dec 23. [19191908 ]
- ATSDR - Agency for Toxic Substances and Disease Registry (2002). Toxicological profile for beryllium. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- General Function:
- Peptide antigen binding
- Specific Function:
- Binds peptides derived from antigens that access the endocytic route of antigen presenting cells (APC) and presents them on the cell surface for recognition by the CD4 T-cells. The peptide binding cleft accommodates peptides of 10-30 residues. The peptides presented by MHC class II molecules are generated mostly by degradation of proteins that access the endocytic route, where they are processed by lysosomal proteases and other hydrolases. Exogenous antigens that have been endocytosed by the APC are thus readily available for presentation via MHC II molecules, and for this reason this antigen presentation pathway is usually referred to as exogenous. As membrane proteins on their way to degradation in lysosomes as part of their normal turn-over are also contained in the endosomal/lysosomal compartments, exogenous antigens must compete with those derived from endogenous components. Autophagy is also a source of endogenous peptides, autophagosomes constitutively fuse with MHC class II loading compartments. In addition to APCs, other cells of the gastrointestinal tract, such as epithelial cells, express MHC class II molecules and CD74 and act as APCs, which is an unusual trait of the GI tract. To produce a MHC class II molecule that presents an antigen, three MHC class II molecules (heterodimers of an alpha and a beta chain) associate with a CD74 trimer in the ER to form a heterononamer. Soon after the entry of this complex into the endosomal/lysosomal system where antigen processing occurs, CD74 undergoes a sequential degradation by various proteases, including CTSS and CTSL, leaving a small fragment termed CLIP (class-II-associated invariant chain peptide). The removal of CLIP is facilitated by HLA-DM via direct binding to the alpha-beta-CLIP complex so that CLIP is released. HLA-DM stabilizes MHC class II molecules until primary high affinity antigenic peptides are bound. The MHC II molecule bound to a peptide is then transported to the cell membrane surface. In B-cells, the interaction between HLA-DM and MHC class II molecules is regulated by HLA-DO. Primary dendritic cells (DCs) also to express HLA-DO. Lysosomal microenvironment has been implicated in the regulation of antigen loading into MHC II molecules, increased acidification produces increased proteolysis and efficient peptide loading.
- Gene Name:
- HLA-DPB1
- Uniprot ID:
- P04440
- Molecular Weight:
- 29159.195 Da
References
- Amicosante M, Berretta F, Dweik R, Saltini C: Role of high-affinity HLA-DP specific CLIP-derived peptides in beryllium binding to the HLA-DPGlu69 berylliosis-associated molecules and presentation to beryllium-sensitized T cells. Immunology. 2009 Sep;128(1 Suppl):e462-70. doi: 10.1111/j.1365-2567.2008.03000.x. Epub 2008 Dec 23. [19191908 ]
- ATSDR - Agency for Toxic Substances and Disease Registry (2002). Toxicological profile for beryllium. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]