| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-06-22 16:08:30 UTC |
|---|
| Update Date | 2014-12-24 20:24:32 UTC |
|---|
| Accession Number | T3D1725 |
|---|
| Identification |
|---|
| Common Name | Bronidox |
|---|
| Class | Small Molecule |
|---|
| Description | Bronidox is an organobromide that is a nitrobromo derivative of dioxane. It is used as a stabilizer, surfacant, bactericide, and a preservative in immunology and cosmetics. It is corrosive to metals. Bronidox has been used in cosmetics since the mid-1970s as preservative for shampoos and foam baths. |
|---|
| Compound Type | - Bromide Compound
- Inorganic Compound
- Lachrymator
- Organic Compound
- Organobromide
- Pesticide
- Synthetic Compound
|
|---|
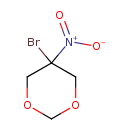
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 5-Brom-5-nitro-1,3-dioxan | | 5-Bromo-5-nitro-1,3-dioxane | | 5-Bromo-5-nitro-m-dioxane | | Bronidox l |
|
|---|
| Chemical Formula | C4H6BrNO4 |
|---|
| Average Molecular Mass | 211.999 g/mol |
|---|
| Monoisotopic Mass | 210.948 g/mol |
|---|
| CAS Registry Number | 30007-47-7 |
|---|
| IUPAC Name | 5-bromo-5-nitro-1,3-dioxane |
|---|
| Traditional Name | 5-bromo-5-nitro-1,3-dioxane |
|---|
| SMILES | [O-][N+](=O)C1(Br)COCOC1 |
|---|
| InChI Identifier | InChI=1S/C4H6BrNO4/c5-4(6(7)8)1-9-3-10-2-4/h1-3H2 |
|---|
| InChI Key | InChIKey=XVBRCOKDZVQYAY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,3-dioxanes. These are organic compounds containing 1,3-dioxane, an aliphatic six-member ring with two oxygen atoms in ring positions 1 and 3. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Dioxanes |
|---|
| Sub Class | 1,3-dioxanes |
|---|
| Direct Parent | 1,3-dioxanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Meta-dioxane
- C-nitro compound
- Organic nitro compound
- Acetal
- Organic oxoazanium
- Allyl-type 1,3-dipolar organic compound
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Oxacycle
- Organooxygen compound
- Organonitrogen compound
- Organobromide
- Organic nitrogen compound
- Organohalogen compound
- Alkyl bromide
- Alkyl halide
- Organopnictogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 60°C | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0190000000-d897d7481e9bf6c3b214 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0900000000-7a213bf457a73fe2834d | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-1900000000-8d36be681c8333da5399 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00kf-0910000000-a1618c08e74f125e9ad0 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-1900000000-ece0e3aaab40b939a994 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02mi-5900000000-3072609e0fd072c5e273 | 2016-08-03 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-002r-9700000000-e7f6debf1376cf9cb084 | 2014-09-20 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (6) ; inhalation (6) ; dermal (6) |
|---|
| Mechanism of Toxicity | Bronidox likely acts as an oxidizing agent that leads to oxidation of free sulfhydryls in essential enzymes. One of the most probable protein targets is the TRPA1 ion channel that is expressed in sensory nerves (trigeminal nerve) of the eyes, nose, mouth and lungs. This compromises their activity and can lead to cell damage, cell death and/or apoptosis. It may also react with amines and amides to form nitrosamines or nitrosamides. Nitrosamines can be carcinogenic. |
|---|
| Metabolism | Bronidox can be metabolized to 2-bromo-2-nitropropane-1,3-diol, a well known nitrosating agent.(6) |
|---|
| Toxicity Values | LD50: 590 mg/kg (mouse, Oral);
LD50: 455 mg/kg (rat, Oral)
LD50: 31 mg/kg (rat, ipr.);
LD50: 2500 µg (mouse, skin);
LD50: 2500 µg (rat, skin) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity (not listed by IARC). (7) |
|---|
| Uses/Sources | Bronidox is used as a stabilizer, surfacant, bactericide, and preservative in immunology and cosmetics. (4) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Can cause significant skin and eye irritation at concentrations >0.1%. A formaldehyde releaser, bromonitrodioxane can cause allergic contact dermatitis in hairdressers and in workers handling cleaners containing bromonitrodioxane. It is neither mutagenic nor teratogenic. |
|---|
| Symptoms | Acute doses cause skin and eye irritation. May cause respiratory tract and mucous membrane irritation if inhaled. Harmful if ingested. May affect behavior/central nervous system (tremor, convulsions, excitement). |
|---|
| Treatment | EYES: irrigate opened eyes for several minutes under running water.
INGESTION: do not induce vomiting. Rinse mouth with water (never give anything by mouth to an unconscious person). Seek immediate medical advice.
SKIN: should be treated immediately by rinsing the affected parts in cold running water for at least 15 minutes, followed by thorough washing with soap and water. If necessary, the person should shower and change contaminated clothing and shoes, and then must seek medical attention.
INHALATION: supply fresh air. If required provide artificial respiration. |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 1807 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 1741 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Bronidox |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D1725.pdf |
|---|
| General References | - Ghannoum M, Thomson M, Bowman W, Al-Khalil S: Mode of action of the antimicrobial compound 5-bromo-5-nitro-1,3-dioxane (bronidox). Folia Microbiol (Praha). 1986;31(1):19-31. [3082729 ]
- Eisenbrand G, Blankart M, Sommer H, Weber B: N-nitrosoalkanolamines in cosmetics. IARC Sci Publ. 1991;(105):238-41. [1855860 ]
- Golomb, BA (1999). A Review of the Scientific Literature As It Pertains to Gulf War Illnesses. Volume 2: Pyridostigmine Bromide. Washington, DC: RAND.
- Wikipedia. Bronidox. Last Updated 19 February 2009. [Link]
- Material Safety Data Sheet. 5-Bromo-5-nitro-1,3-dioxane MSDS [Link]
- International Programme on Chemical Safety (IPCS) INCHEM (1992). Poison Information Monograph for Bromine. [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|