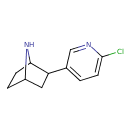| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-03 22:19:13 UTC |
|---|
| Update Date | 2014-12-24 20:25:40 UTC |
|---|
| Accession Number | T3D2530 |
|---|
| Identification |
|---|
| Common Name | Epibatidine |
|---|
| Class | Small Molecule |
|---|
| Description | Epibatidine is a toxin found in certain poisonous frogs (Epipedobates tricolour). It is a powerful analgesic and works by activating nicotinic acetylcholine receptors. (2) |
|---|
| Compound Type | - Amine
- Animal Toxin
- Frog/Toad Toxin
- Natural Compound
- Organic Compound
- Organochloride
|
|---|
| Chemical Structure | |
|---|
| Synonyms | |
|---|
| Chemical Formula | C11H13ClN2 |
|---|
| Average Molecular Mass | 208.687 g/mol |
|---|
| Monoisotopic Mass | 208.077 g/mol |
|---|
| CAS Registry Number | 140111-52-0 |
|---|
| IUPAC Name | 2-(6-chloropyridin-3-yl)-7-azabicyclo[2.2.1]heptane |
|---|
| Traditional Name | epibatidine |
|---|
| SMILES | ClC1=CC=C(C=N1)C1CC2CCC1N2 |
|---|
| InChI Identifier | InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 |
|---|
| InChI Key | InChIKey=NLPRAJRHRHZCQQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as epibatidine analogues. Epibatidine analogues are compounds containing an epibatidine moiety, with a structure characterized by a 2-chloropyridine moiety connected to an 7-azabicyclo[2.2.1]heptane in exo position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Epibatidine analogues |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Epibatidine analogues |
|---|
| Alternative Parents | |
|---|
| Substituents | - Epibatidine-skeleton
- Pyrrolidinylpyridine
- 2-halopyridine
- Aralkylamine
- Aryl chloride
- Aryl halide
- Pyridine
- Heteroaromatic compound
- Pyrrolidine
- Azacycle
- Secondary aliphatic amine
- Secondary amine
- Organoheterocyclic compound
- Amine
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-017l-9800000000-f9be6efcb287e3c5e76d | 2021-09-23 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0190000000-046959b515e82db7e6fb | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-3390000000-8f766845caae11ffc884 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fsi-8900000000-8bd101dee7036183998d | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0190000000-8d4deb0cede3f9c09d4f | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0590000000-9918548508966d5ecabf | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fu-2900000000-26082d9becf367cbadfd | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-535cc7ec7e9fa7416616 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0090000000-535cc7ec7e9fa7416616 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-057l-0920000000-669298ad58e120ed70c3 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-31fc562f3420cf79d8c8 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-1190000000-4c8b54092b4670dea254 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9200000000-bb84d221e09ef401c376 | 2021-10-12 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Injection (sting/bite) (1) ; inhalation (smoking) (3) |
|---|
| Mechanism of Toxicity | Epibatidine works by binding and activating nicotinic acetylcholine receptors. (2) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Epibatidine is a toxin found in certain poisonous frogs (Epipedobates tricolour). (2) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Epibatidine is neurotoxic. It may also cause receptor blocks at neuromuscular junctions, causing respiratory paralysis and death. It is also a powerful analgesic. (2) |
|---|
| Symptoms | Epibatidine is neurotoxic. It may also cause receptor blocks at neuromuscular junctions, causing respiratory paralysis and death. It is also a powerful analgesic. (2) |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB07720 |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 1204 |
|---|
| ChEMBL ID | CHEMBL6623 |
|---|
| ChemSpider ID | 1167 |
|---|
| KEGG ID | C11690 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | C082748 |
|---|
| Stitch ID | Epibatidine |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Csaba Sz antay, Zsuzsanna B. Kardos, Istv an Moldvai, Eszter T. Major, Csaba Sz antay, Jr., Attila M andi, G abor Blask o, Gyula Simig, Gy orgyi Lax, S andor Drabant, Tamas Sz all asi, M arton Fekete, G abor Gigler, “Process for the preparation of epibatidine.” U.S. Patent US5545741, issued March, 1994. |
|---|
| MSDS | Not Available |
|---|
| General References | - Chiba K, Trevor A, Castagnoli N Jr: Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem Biophys Res Commun. 1984 Apr 30;120(2):574-8. [6428396 ]
- Wikipedia. Epibatidine. Last Updated 31 May 2009. [Link]
- Wikipedia. Frog. Last Updated 10 August 2009. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|