| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-05 17:25:24 UTC |
|---|
| Update Date | 2014-12-24 20:25:44 UTC |
|---|
| Accession Number | T3D2587 |
|---|
| Identification |
|---|
| Common Name | Maitotoxin |
|---|
| Class | Small Molecule |
|---|
| Description | Maitotoxin (MTX) is an extremely potent toxin produced by a species of dinoflagellates (Gambierdiscus toxicus). It activates calcium permeable, non-selective cation channels, leading to an increase in levels of cytosolic calcium ions. Ultimately, a cell death cascade is activated, resulting in membrane blebbing and eventually cell lysis. (2) |
|---|
| Compound Type | - Animal Toxin
- Ester
- Ether
- Marine Toxin
- Natural Compound
- Organic Compound
|
|---|
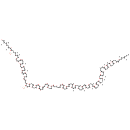
| Chemical Structure | |
|---|
| Synonyms | |
|---|
| Chemical Formula | C164H256Na2O68S2 |
|---|
| Average Molecular Mass | 3425.856 g/mol |
|---|
| Monoisotopic Mass | 3423.581 g/mol |
|---|
| CAS Registry Number | 59392-53-9 |
|---|
| IUPAC Name | disodium (2S,3R,4R,4aS,5aR,6aS,7aS,8R,9R,10R,11aR,12R,12aR,13aS,14aR)-10-[(2R,3R,4R,4aS,6S,7R,8aS)-6-[(1R,3R)-4-[(2S,3R,4R,4aS,6R,7R,8aS)-6-[(1R,3S,5R,7S,9R,10R,12R,13S,14S,16R,19S,21R,23S,25S,28R,30S)-25-[(1S,3R,5S,7R,9S,11S,14R,16S,18R,20S,21Z,24R,26S,28R,30S,32R,34R,35R,37S,39R,42S,44R)-11-[(1S,2R,4R,5S)-1,2-dihydroxy-4,5-dimethyloct-7-en-1-yl]-35-hydroxy-14,16,18,32,34,39,42,44-octamethyl-2,6,10,15,19,25,29,33,38,43-decaoxadecacyclo[22.21.0.0³,²⁰.0⁵,¹⁸.0⁷,¹⁶.0⁹,¹⁴.0²⁶,⁴⁴.0²⁸,⁴².0³⁰,³⁹.0³²,³⁷]pentatetracont-21-en-34-yl]-9,13-dihydroxy-3,7,14,19,30-pentamethyl-2,6,11,15,20,24,29-heptaoxaheptacyclo[17.12.0.0³,¹⁶.0⁵,¹⁴.0⁷,¹².0²¹,³⁰.0²³,²⁸]hentriacontan-10-yl]-3,4,7-trihydroxy-octahydropyrano[3,2-b]pyran-2-yl]-1,3-dihydroxybutyl]-3,4,7-trihydroxy-octahydropyrano[3,2-b]pyran-2-yl]-2-[(2S,3R)-2,3-dihydroxy-3-[(1S,3R,5S,6S,7R,8S,10R,11R,13S,15R,17S,19R,21R,22S,24S,25S,26R)-6,7,11,21,25-pentahydroxy-13,17-dimethyl-8-[(2R,3R,4R,7S,8R,9R,11R,13E)-3,8,11,15-tetrahydroxy-4,9,13-trimethyl-12-methylidene-7-(sulfonatooxy)pentadec-13-en-2-yl]-4,9,14,18,23,27-hexaoxahexacyclo[13.12.0.0³,¹³.0⁵,¹⁰.0¹⁷,²⁶.0¹⁹,²⁴]heptacosan-22-yl]propyl]-4,8,9,12-tetrahydroxy-hexadecahydro-2H-1,5,7,11,13-pentaoxapentacen-3-yl sulfate |
|---|
| Traditional Name | disodium (2S,3R,4R,4aS,5aR,6aS,7aS,8R,9R,10R,11aR,12R,12aR,13aS,14aR)-10-[(2R,3R,4R,4aS,6S,7R,8aS)-6-[(1R,3R)-4-[(2S,3R,4R,4aS,6R,7R,8aS)-6-[(1R,3S,5R,7S,9R,10R,12R,13S,14S,16R,19S,21R,23S,25S,28R,30S)-25-[(1S,3R,5S,7R,9S,11S,14R,16S,18R,20S,21Z,24R,26S,28R,30S,32R,34R,35R,37S,39R,42S,44R)-11-[(1S,2R,4R,5S)-1,2-dihydroxy-4,5-dimethyloct-7-en-1-yl]-35-hydroxy-14,16,18,32,34,39,42,44-octamethyl-2,6,10,15,19,25,29,33,38,43-decaoxadecacyclo[22.21.0.0³,²⁰.0⁵,¹⁸.0⁷,¹⁶.0⁹,¹⁴.0²⁶,⁴⁴.0²⁸,⁴².0³⁰,³⁹.0³²,³⁷]pentatetracont-21-en-34-yl]-9,13-dihydroxy-3,7,14,19,30-pentamethyl-2,6,11,15,20,24,29-heptaoxaheptacyclo[17.12.0.0³,¹⁶.0⁵,¹⁴.0⁷,¹².0²¹,³⁰.0²³,²⁸]hentriacontan-10-yl]-3,4,7-trihydroxy-octahydropyrano[3,2-b]pyran-2-yl]-1,3-dihydroxybutyl]-3,4,7-trihydroxy-octahydropyrano[3,2-b]pyran-2-yl]-2-[(2S,3R)-2,3-dihydroxy-3-[(1S,3R,5S,6S,7R,8S,10R,11R,13S,15R,17S,19R,21R,22S,24S,25S,26R)-6,7,11,21,25-pentahydroxy-13,17-dimethyl-8-[(2R,3R,4R,7S,8R,9R,11R,13E)-3,8,11,15-tetrahydroxy-4,9,13-trimethyl-12-methylidene-7-(sulfonatooxy)pentadec-13-en-2-yl]-4,9,14,18,23,27-hexaoxahexacyclo[13.12.0.0³,¹³.0⁵,¹⁰.0¹⁷,²⁶.0¹⁹,²⁴]heptacosan-22-yl]propyl]-4,8,9,12-tetrahydroxy-hexadecahydro-2H-1,5,7,11,13-pentaoxapentacen-3-yl sulfate |
|---|
| SMILES | [Na+].[Na+].[H]\C(CO)=C(\C)C(=C)[C@]([H])(O)C[C@@]([H])(C)[C@@]([H])(O)[C@]([H])(CC[C@@]([H])(C)[C@@]([H])(O)[C@@]([H])(C)[C@]1([H])O[C@@]2([H])[C@@]([H])(O[C@]3([H])C[C@]4([H])O[C@]5([H])[C@@]([H])(O)[C@]6([H])O[C@]([H])([C@]([H])(O)[C@@]([H])(O)C[C@]7([H])O[C@]8([H])C[C@]9([H])O[C@@]%10([H])[C@]([H])(C[C@@]9([H])O[C@@]8([H])[C@@]([H])(O)[C@@]7([H])OS([O-])(=O)=O)O[C@@]7([H])[C@]([H])(O)[C@@]([H])(O)[C@@]([H])(O[C@]7([H])[C@]%10([H])O)[C@]7([H])O[C@@]8([H])C[C@@]([H])(O)[C@@]([H])(O[C@@]8([H])[C@]([H])(O)[C@@]7([H])O)[C@]([H])(O)C[C@@]([H])(O)C[C@]7([H])O[C@@]8([H])C[C@@]([H])(O)[C@@]([H])(O[C@@]8([H])[C@]([H])(O)[C@@]7([H])O)[C@]7([H])O[C@]8([H])[C@]([H])(O)[C@]9(C)O[C@]%10([H])CC[C@]%11(C)O[C@]%12([H])C[C@]%13([H])O[C@@]([H])(CC[C@@]%13([H])O[C@@]%12(C)C[C@@]%11([H])O[C@@]%10(C)C[C@@]9([H])O[C@@]8(C)C[C@@]7([H])O)[C@]7(C)O[C@]8(C)C[C@]9([H])O[C@]%10([H])C[C@]%11([H])O[C@]%12([H])C\C([H])=C([H])/[C@]%13([H])O[C@]%14(C)C[C@]%15(C)O[C@]%16(C)CC[C@]([H])(O[C@@]%16([H])C[C@@]%15([H])O[C@@]%14([H])C[C@@]%13([H])O[C@@]%12([H])C[C@@]%11(C)O[C@@]%10(C)CC[C@@]9(C)O[C@@]8([H])C[C@@]7([H])O)[C@@]([H])(O)[C@]([H])(O)C[C@@]([H])(C)[C@@]([H])(C)CC=C)[C@]([H])(O)C[C@@]6([H])O[C@@]5(C)C[C@@]4([H])O[C@@]3(C)C[C@@]2([H])O)[C@@]([H])(O)[C@@]1([H])O)OS([O-])(=O)=O |
|---|
| InChI Identifier | InChI=1S/C164H258O68S2.2Na/c1-24-26-65(2)68(5)41-74(168)117(179)85-33-36-152(11)106(203-85)55-109-162(21,231-152)64-161(20)105(210-109)51-89-83(220-161)28-25-27-82-99(199-89)59-157(16)108(202-82)56-107-153(12,230-157)39-38-151(10)112(211-107)61-158(17)111(224-151)54-101(176)163(22,232-158)103-32-31-84-90(204-103)53-110-156(15,219-84)62-113-150(9,223-110)37-34-102-155(14,225-113)63-114-164(23,227-102)147(192)149-159(18,226-114)58-81(175)134(218-149)133-79(173)47-93-136(216-133)120(182)119(181)92(200-93)44-72(166)43-76(170)131-77(171)46-94-137(214-131)122(184)124(186)143(207-94)145-126(188)125(187)144-146(217-145)128(190)139-97(208-144)50-88-87(206-139)49-96-138(205-88)127(189)141(229-234(196,197)198)95(201-96)45-75(169)118(180)132-78(172)48-98-140(215-132)129(191)148-160(19,221-98)60-100-91(209-148)52-104-154(13,222-100)57-80(174)135-142(212-104)123(185)121(183)130(213-135)71(8)115(177)67(4)29-30-86(228-233(193,194)195)116(178)69(6)42-73(167)70(7)66(3)35-40-165;;/h24-25,28,35,65,67-69,71-149,165-192H,1,7,26-27,29-34,36-64H2,2-6,8-23H3,(H,193,194,195)(H,196,197,198);;/q;2*+1/p-2/b28-25-,66-35+;;/t65-,67+,68+,69+,71+,72+,73+,74+,75-,76+,77+,78+,79+,80+,81+,82+,83-,84+,85-,86-,87-,88+,89+,90-,91-,92-,93-,94-,95-,96+,97-,98+,99-,100+,101+,102+,103-,104+,105-,106-,107+,108-,109+,110+,111-,112-,113+,114+,115+,116+,117-,118+,119-,120+,121+,122+,123-,124+,125+,126+,127+,128+,129-,130-,131-,132-,133+,134+,135+,136+,137+,138+,139-,140+,141-,142-,143+,144-,145+,146+,147-,148+,149+,150-,151+,152+,153-,154-,155-,156-,157+,158+,159-,160-,161+,162-,163+,164+;;/m0../s1 |
|---|
| InChI Key | InChIKey=NWQUHAJRFNRIIU-DVGFTKJRSA-L |
|---|
| Chemical Taxonomy |
|---|
| Classification | Not classified |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Acrosome
- Actin Cytoskeleton
- Actin Filament
- Cell surface
- Cytoskeleton
- Cytosol
- Endoplasmic reticulum
- Extracellular
- Membrane
- Microtubule
- Mitochondrion
- Nuclear Matrix
- Plasma Membrane
- Secretory Granule
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000900000-7ef155cf949e833ed523 | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0000900000-7ef155cf949e833ed523 | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-0000900000-7ef155cf949e833ed523 | 2019-02-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Injection (sting/bite) (3) |
|---|
| Mechanism of Toxicity | Maitotoxin activates calcium permeable, non-selective cation channels, leading to an increase in levels of cytosolic calcium ions. It is thought that maitotoxin leads to the formation of pores on these ion channels. Ultimately, a cell death cascade is activated, resulting in membrane blebbing and eventually cell lysis. Maitotoxin is also known to activate cytosolic calcium-activated proteases calpain-1 and calpain-2, contributing to necrosis. (2) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | LD50: 50 ug/kg (Mouse) (2) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Maitotoxin is an extremely potent toxin produced by a species of dinoflagellates (Gambierdiscus toxicus). (2) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Maitotoxin is a potent cytotoxin. (2) |
|---|
| Symptoms | Toxin from Gambierdiscus toxicus can cause a type of food poisoning called ciguatera. Hallmark symptoms of ciguatera include gastrointestinal and neurological effects. Gastrointestinal symptoms include nausea, vomiting, and diarrhea usually followed by neurological symptoms such as headaches, muscle aches, paresthesia, numbness, ataxia, and hallucinations. Severe cases of ciguatera can also result in cold allodynia. (1) |
|---|
| Treatment | Ciguatera poisoning is treated with supportive care. Some medications such as the use of Amitriptyline may reduce some symptoms of ciguatera, such as fatigue and paresthesia, although benefit does not occur in every case. Also used are steroids and vitamin supplements, but these merely support the body's recovery rather than directly reducing the toxic effects. (1) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 71460273 |
|---|
| ChEMBL ID | CHEMBL2152261 |
|---|
| ChemSpider ID | 10477103 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | C020394 |
|---|
| Stitch ID | Maitotoxin |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Maitotoxin |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - Wikipedia. Ciguatera. Last Updated 3 August 2009. [Link]
- Wikipedia. Maitotoxin. Last Updated 10 June 2009. [Link]
- Wikipedia. Mollusca. Last Updated 5 August 2009. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|