You are using an unsupported browser. Please upgrade your browser to a newer version to get the best experience on Toxin, Toxin Target Database.
Buspirone (T3D2807)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-07-21 20:27:01 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:25:51 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D2807 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Buspirone | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Buspirone is only found in individuals that have used or taken this drug. It is an anxiolytic agent and a serotonin receptor agonist belonging to the azaspirodecanedione class of compounds. Its structure is unrelated to those of the benzodiazepines, but it has an efficacy comparable to diazepam. Buspirone binds to 5-HT type 1A serotonin receptors on presynaptic neurons in the dorsal raphe and on postsynaptic neurons in the hippocampus, thus inhibiting the firing rate of 5-HT-containing neurons in the dorsal raphe. Buspirone also binds at dopamine type 2 (DA2) receptors, blocking presynaptic dopamine receptors. Buspirone increases firing in the locus ceruleus, an area of brain where norepinephrine cell bodies are found in high concentration. The net result of buspirone actions is that serotonergic activity is suppressed while noradrenergic and dopaminergic cell firing is enhanced. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
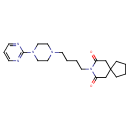
| Chemical Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C21H31N5O2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 385.503 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 385.248 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 36505-84-7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | 8-{4-[4-(pyrimidin-2-yl)piperazin-1-yl]butyl}-8-azaspiro[4.5]decane-7,9-dione | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | buspirone | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | O=C1CC2(CCCC2)CC(=O)N1CCCCN1CCN(CC1)C1=NC=CC=N1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C21H31N5O2/c27-18-16-21(6-1-2-7-21)17-19(28)26(18)11-4-3-10-24-12-14-25(15-13-24)20-22-8-5-9-23-20/h5,8-9H,1-4,6-7,10-17H2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=QWCRAEMEVRGPNT-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as n-arylpiperazines. These are organic compounds containing a piperazine ring where the nitrogen ring atom carries an aryl group. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Diazinanes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Piperazines | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | N-arylpiperazines | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | White powder. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Oral. Rapidly absorbed in man. Bioavailability is low and variable (approximately 5%) due to extensive first pass metabolism. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Buspirone binds to 5-HT type 1A serotonin receptors on presynaptic neurons in the dorsal raphe and on postsynaptic neurons in the hippocampus, thus inhibiting the firing rate of 5-HT-containing neurons in the dorsal raphe. Buspirone also binds at dopamine type 2 (DA2) receptors, blocking presynaptic dopamine receptors. Buspirone increases firing in the locus ceruleus, an area of brain where norepinephrine cell bodies are found in high concentration. The net result of buspirone actions is that serotonergic activity is suppressed while noradrenergic and dopaminergic cell firing is enhanced. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Metabolized hepatically, primarily by oxidation by cytochrome P450 3A4 producing several hydroxylated derivatives and a pharmacologically active metabolite, 1-pyrimidinylpiperazine (1-PP) Route of Elimination: In a single-dose study using 14C-labeled buspirone, 29% to 63% of the dose was excreted in the urine within 24 hours, primarily as metabolites; fecal excretion accounted for 18% to 38% of the dose. Half Life: 2-3 hours (although the action of a single dose is much longer than the short halflife indicates). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | LD50: 136 mg/kg (Oral, rat) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | For the management of anxiety disorders or the short-term relief of the symptoms of anxiety, and also as an augmention of SSRI-treatment against depression. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Symptoms of overdose include dizziness, drowsiness, nausea or vomiting, severe stomach upset, and unusually small pupils. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | General symptomatic and supportive measures should be used along with immediate gastric lavage. Respiration, pulse, and blood pressure should be monitored as in all cases of drug overdosage. No specific antidote is known to buspirone, and dialyzability of buspirone has not been determined. (2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | DB00490 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB14633 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 2477 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | CHEMBL49 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 2383 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | C06861 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | 608902 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 3223 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Buspirone | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Buspirone | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Link | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors, such as adenylate cyclase. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling inhibits adenylate cyclase activity and activates a phosphatidylinositol-calcium second messenger system that regulates the release of Ca(2+) ions from intracellular stores. Plays a role in the regulation of 5-hydroxytryptamine release and in the regulation of dopamine and 5-hydroxytryptamine metabolism. Plays a role in the regulation of dopamine and 5-hydroxytryptamine levels in the brain, and thereby affects neural activity, mood and behavior. Plays a role in the response to anxiogenic stimuli.
- Gene Name:
- HTR1A
- Uniprot ID:
- P08908
- Molecular Weight:
- 46106.335 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0031 uM | Not Available | BindingDB 50001859 |
| Inhibitory | 0.00398 uM | Not Available | BindingDB 50001859 |
| Inhibitory | 0.0066 uM | Not Available | BindingDB 50001859 |
| Inhibitory | 0.015 uM | Not Available | BindingDB 50001859 |
| Inhibitory | 0.01513 uM | Not Available | BindingDB 50001859 |
| Inhibitory | 0.017 uM | Not Available | BindingDB 50001859 |
| Inhibitory | 0.01905 uM | Not Available | BindingDB 50001859 |
| Inhibitory | 0.02 uM | Not Available | BindingDB 50001859 |
| Inhibitory | 0.021 uM | Not Available | BindingDB 50001859 |
| Inhibitory | 0.0211 uM | Not Available | BindingDB 50001859 |
| Inhibitory | 0.02137 uM | Not Available | BindingDB 50001859 |
| Inhibitory | 0.024 uM | Not Available | BindingDB 50001859 |
| Inhibitory | 0.03 uM | Not Available | BindingDB 50001859 |
| Inhibitory | 0.07762 uM | Not Available | BindingDB 50001859 |
| Inhibitory | 0.212 uM | Not Available | BindingDB 50001859 |
| Inhibitory | 0.21379 uM | Not Available | BindingDB 50001859 |
| IC50 | 0.025 uM | Not Available | BindingDB 50001859 |
References
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. [11752352 ]
- de Boer SF, Lesourd M, Mocaer E, Koolhaas JM: Selective antiaggressive effects of alnespirone in resident-intruder test are mediated via 5-hydroxytryptamine1A receptors: A comparative pharmacological study with 8-hydroxy-2-dipropylaminotetralin, ipsapirone, buspirone, eltoprazine, and WAY-100635. J Pharmacol Exp Ther. 1999 Mar;288(3):1125-33. [10027850 ]
- Rehman J, Kaynan A, Christ G, Valcic M, Maayani S, Melman A: Modification of sexual behavior of Long-Evans male rats by drugs acting on the 5-HT1A receptor. Brain Res. 1999 Mar 13;821(2):414-25. [10064829 ]
- Liang KC: Pre- or post-training injection of buspirone impaired retention in the inhibitory avoidance task: involvement of amygdala 5-HT1A receptors. Eur J Neurosci. 1999 May;11(5):1491-500. [10215901 ]
- Becker C, Hamon M, Benoliel JJ: Prevention by 5-HT1A receptor agonists of restraint stress- and yohimbine-induced release of cholecystokinin in the frontal cortex of the freely moving rat. Neuropharmacology. 1999 Apr;38(4):525-32. [10221756 ]
- Dupuis DS, Tardif S, Wurch T, Colpaert FC, Pauwels PJ: Modulation of 5-HT1A receptor signalling by point-mutation of cysteine351 in the rat Galpha(o) protein. Neuropharmacology. 1999 Jul;38(7):1035-41. [10428422 ]
- Tandon M, O'Donnell MM, Porte A, Vensel D, Yang D, Palma R, Beresford A, Ashwell MA: The design and preparation of metabolically protected new arylpiperazine 5-HT1A ligands. Bioorg Med Chem Lett. 2004 Apr 5;14(7):1709-12. [15026055 ]
- Romero AG, Leiby JA, McCall RB, Piercey MF, Smith MW, Han F: Novel 2-substituted tetrahydro-3H-benz[e]indolamines: highly potent and selective agonists acting at the 5-HT1A receptor as possible anxiolytics and antidepressants. J Med Chem. 1993 Jul 23;36(15):2066-74. [8101876 ]
- Boess FG, Martin IL: Molecular biology of 5-HT receptors. Neuropharmacology. 1994 Mar-Apr;33(3-4):275-317. [7984267 ]
- Moon MW, Morris JK, Heier RF, Chidester CG, Hoffmann WE, Piercey MF, Althaus JS, Von Voigtlander PF, Evans DL, Figur LM, et al.: Dopaminergic and serotonergic activities of imidazoquinolinones and related compounds. J Med Chem. 1992 Mar 20;35(6):1076-92. [1348089 ]
- Dounay AB, Barta NS, Bikker JA, Borosky SA, Campbell BM, Crawford T, Denny L, Evans LM, Gray DL, Lee P, Lenoir EA, Xu W: Synthesis and pharmacological evaluation of aminopyrimidine series of 5-HT1A partial agonists. Bioorg Med Chem Lett. 2009 Feb 15;19(4):1159-63. doi: 10.1016/j.bmcl.2008.12.087. Epub 2008 Dec 25. [19147349 ]
- Zajdel P, Subra G, Bojarski AJ, Duszynska B, Pawlowski M, Martinez J: Arylpiperazines with N-acylated amino acids as 5-HT1A receptor ligands. Bioorg Med Chem Lett. 2006 Jul 1;16(13):3406-10. Epub 2006 May 3. [16677812 ]
- Becker OM, Dhanoa DS, Marantz Y, Chen D, Shacham S, Cheruku S, Heifetz A, Mohanty P, Fichman M, Sharadendu A, Nudelman R, Kauffman M, Noiman S: An integrated in silico 3D model-driven discovery of a novel, potent, and selective amidosulfonamide 5-HT1A agonist (PRX-00023) for the treatment of anxiety and depression. J Med Chem. 2006 Jun 1;49(11):3116-35. [16722631 ]
- Liu Z, Zhang H, Ye N, Zhang J, Wu Q, Sun P, Li L, Zhen X, Zhang A: Synthesis of dihydrofuroaporphine derivatives: identification of a potent and selective serotonin 5-HT 1A receptor agonist. J Med Chem. 2010 Feb 11;53(3):1319-28. doi: 10.1021/jm9015763. [20041669 ]
- Bang-Andersen B, Ruhland T, Jorgensen M, Smith G, Frederiksen K, Jensen KG, Zhong H, Nielsen SM, Hogg S, Mork A, Stensbol TB: Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem. 2011 May 12;54(9):3206-21. doi: 10.1021/jm101459g. Epub 2011 Apr 12. [21486038 ]
- Sundaram H, Newman-Tancredi A, Strange PG: Characterization of recombinant human serotonin 5HT1A receptors expressed in Chinese hamster ovary cells. [3H]spiperone discriminates between the G-protein-coupled and -uncoupled forms. Biochem Pharmacol. 1993 Mar 9;45(5):1003-9. [8461029 ]
- Hamon M, Lanfumey L, el Mestikawy S, Boni C, Miquel MC, Bolanos F, Schechter L, Gozlan H: The main features of central 5-HT1 receptors. Neuropsychopharmacology. 1990 Oct-Dec;3(5-6):349-60. [2078271 ]
- General Function:
- Potassium channel regulator activity
- Specific Function:
- Dopamine receptor whose activity is mediated by G proteins which inhibit adenylyl cyclase.
- Gene Name:
- DRD2
- Uniprot ID:
- P14416
- Molecular Weight:
- 50618.91 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.013 uM | Not Available | BindingDB 50001859 |
| Inhibitory | 0.09 uM | Not Available | BindingDB 50001859 |
References
- Malt EA, Olafsson S, Aakvaag A, Lund A, Ursin H: Altered dopamine D2 receptor function in fibromyalgia patients: a neuroendocrine study with buspirone in women with fibromyalgia compared to female population based controls. J Affect Disord. 2003 Jun;75(1):77-82. [12781354 ]
- Boido A, Boido CC, Sparatore F: Synthesis and pharmacological evaluation of aryl/heteroaryl piperazinyl alkyl benzotriazoles as ligands for some serotonin and dopamine receptor subtypes. Farmaco. 2001 Apr;56(4):263-75. [11421254 ]
- Nader MA, Hannemann M: Interactions of buspirone or gepirone with nicotine on schedule-controlled behavior of pigeons. Behav Pharmacol. 1993 Jun;4(3):263-268. [11224194 ]
- Pache DM, Fernandez-Perez S, Sewell RD: Buspirone differentially modifies short-term memory function in a combined delayed matching/non-matching to position task. Eur J Pharmacol. 2003 Sep 23;477(3):205-11. [14522358 ]
- Fernandez-Perez S, Pache DM, Sewell RD: Co-administration of fluoxetine and WAY100635 improves short-term memory function. Eur J Pharmacol. 2005 Oct 17;522(1-3):78-83. Epub 2005 Oct 7. [16214127 ]
- Romero AG, Leiby JA, McCall RB, Piercey MF, Smith MW, Han F: Novel 2-substituted tetrahydro-3H-benz[e]indolamines: highly potent and selective agonists acting at the 5-HT1A receptor as possible anxiolytics and antidepressants. J Med Chem. 1993 Jul 23;36(15):2066-74. [8101876 ]
- Becker OM, Dhanoa DS, Marantz Y, Chen D, Shacham S, Cheruku S, Heifetz A, Mohanty P, Fichman M, Sharadendu A, Nudelman R, Kauffman M, Noiman S: An integrated in silico 3D model-driven discovery of a novel, potent, and selective amidosulfonamide 5-HT1A agonist (PRX-00023) for the treatment of anxiety and depression. J Med Chem. 2006 Jun 1;49(11):3116-35. [16722631 ]
- Kawakubo H, Takagi S, Yamaura Y, Katoh S, Ishimoto Y, Nagatani T, Mochizuki D, Kamata T, Sasaki Y: (R)-1,2,3,4-tetrahydro[1]benzothieno[2,3-c]pyridines: novel optically active compounds with strong 5-HT1A receptor binding ability exhibiting anticonflict activity and lessening of memory impairment. J Med Chem. 1993 Nov 12;36(23):3526-32. [7902439 ]
- General Function:
- Virus receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances, including mescaline, psilocybin, 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) and lysergic acid diethylamide (LSD). Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling activates phospholipase C and a phosphatidylinositol-calcium second messenger system that modulates the activity of phosphatidylinositol 3-kinase and promotes the release of Ca(2+) ions from intracellular stores. Affects neural activity, perception, cognition and mood. Plays a role in the regulation of behavior, including responses to anxiogenic situations and psychoactive substances. Plays a role in intestinal smooth muscle contraction, and may play a role in arterial vasoconstriction.(Microbial infection) Acts as a receptor for human JC polyomavirus/JCPyV.
- Gene Name:
- HTR2A
- Uniprot ID:
- P28223
- Molecular Weight:
- 52602.58 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.13803 uM | Not Available | BindingDB 50001859 |
| Inhibitory | 0.851 uM | Not Available | BindingDB 50001859 |
References
- Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K, Fontana DJ, Trinh J, Rocha CL, Dawson MW, Flippin LA, Eglen RM: RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology. 1997 Apr-May;36(4-5):621-9. [9225287 ]
- Bojarski AJ, Kuran B, Kossakowski J, Koziol A, Jagiello-Wojtowicz E, Chodkowska A: Synthesis and serotonin receptor activity of the arylpiperazine alkyl/propoxy derivatives of new azatricycloundecanes. Eur J Med Chem. 2009 Jan;44(1):152-64. doi: 10.1016/j.ejmech.2008.03.019. Epub 2008 Apr 8. [18486277 ]
- General Function:
- Opioid receptor activity
- Specific Function:
- Functions in lipid transport from the endoplasmic reticulum and is involved in a wide array of cellular functions probably through regulation of the biogenesis of lipid microdomains at the plasma membrane. Involved in the regulation of different receptors it plays a role in BDNF signaling and EGF signaling. Also regulates ion channels like the potassium channel and could modulate neurotransmitter release. Plays a role in calcium signaling through modulation together with ANK2 of the ITP3R-dependent calcium efflux at the endoplasmic reticulum. Plays a role in several other cell functions including proliferation, survival and death. Originally identified for its ability to bind various psychoactive drugs it is involved in learning processes, memory and mood alteration (PubMed:16472803, PubMed:9341151). Necessary for proper mitochondrial axonal transport in motor neurons, in particular the retrograde movement of mitochondria (By similarity).
- Gene Name:
- SIGMAR1
- Uniprot ID:
- Q99720
- Molecular Weight:
- 25127.52 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 0.036 uM | Not Available | BindingDB 50001859 |
| IC50 | 0.263 uM | Not Available | BindingDB 50001859 |
References
- Myers AM, Charifson PS, Owens CE, Kula NS, McPhail AT, Baldessarini RJ, Booth RG, Wyrick SD: Conformational analysis, pharmacophore identification, and comparative molecular field analysis of ligands for the neuromodulatory sigma 3 receptor. J Med Chem. 1994 Nov 25;37(24):4109-17. [7990111 ]
- Perrone R, Berardi F, Colabufo NA, Leopoldo M, Tortorella V, Fiorentini F, Olgiati V, Ghiglieri A, Govoni S: High affinity and selectivity on 5-HT1A receptor of 1-aryl-4-[1-tetralin)alkyl]piperazines. 2. J Med Chem. 1995 Mar 17;38(6):942-9. [7699710 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various ergot alkaloid derivatives and psychoactive substances. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling activates a phosphatidylinositol-calcium second messenger system that modulates the activity of phosphatidylinositol 3-kinase and down-stream signaling cascades and promotes the release of Ca(2+) ions from intracellular stores. Plays a role in the regulation of dopamine and 5-hydroxytryptamine release, 5-hydroxytryptamine uptake and in the regulation of extracellular dopamine and 5-hydroxytryptamine levels, and thereby affects neural activity. May play a role in the perception of pain. Plays a role in the regulation of behavior, including impulsive behavior. Required for normal proliferation of embryonic cardiac myocytes and normal heart development. Protects cardiomyocytes against apoptosis. Plays a role in the adaptation of pulmonary arteries to chronic hypoxia. Plays a role in vasoconstriction. Required for normal osteoblast function and proliferation, and for maintaining normal bone density. Required for normal proliferation of the interstitial cells of Cajal in the intestine.
- Gene Name:
- HTR2B
- Uniprot ID:
- P41595
- Molecular Weight:
- 54297.41 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.21379 uM | Not Available | BindingDB 50001859 |
References
- Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K, Fontana DJ, Trinh J, Rocha CL, Dawson MW, Flippin LA, Eglen RM: RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology. 1997 Apr-May;36(4-5):621-9. [9225287 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances, including ergot alkaloid derivatives, 1-2,5,-dimethoxy-4-iodophenyl-2-aminopropane (DOI) and lysergic acid diethylamide (LSD). Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling activates a phosphatidylinositol-calcium second messenger system that modulates the activity of phosphatidylinositol 3-kinase and down-stream signaling cascades and promotes the release of Ca(2+) ions from intracellular stores. Regulates neuronal activity via the activation of short transient receptor potential calcium channels in the brain, and thereby modulates the activation of pro-opiomelacortin neurons and the release of CRH that then regulates the release of corticosterone. Plays a role in the regulation of appetite and eating behavior, responses to anxiogenic stimuli and stress. Plays a role in insulin sensitivity and glucose homeostasis.
- Gene Name:
- HTR2C
- Uniprot ID:
- P28335
- Molecular Weight:
- 51820.705 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.48977 uM | Not Available | BindingDB 50001859 |
References
- Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K, Fontana DJ, Trinh J, Rocha CL, Dawson MW, Flippin LA, Eglen RM: RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology. 1997 Apr-May;36(4-5):621-9. [9225287 ]
- General Function:
- Voltage-gated potassium channel activity
- Specific Function:
- This is one of the several different receptors for 5-hydroxytryptamine (serotonin), a biogenic hormone that functions as a neurotransmitter, a hormone, and a mitogen. This receptor is a ligand-gated ion channel, which when activated causes fast, depolarizing responses in neurons. It is a cation-specific, but otherwise relatively nonselective, ion channel.
- Gene Name:
- HTR3A
- Uniprot ID:
- P46098
- Molecular Weight:
- 55279.835 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | >10 uM | Not Available | BindingDB 50001859 |
References
- Bang-Andersen B, Ruhland T, Jorgensen M, Smith G, Frederiksen K, Jensen KG, Zhong H, Nielsen SM, Hogg S, Mork A, Stensbol TB: Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem. 2011 May 12;54(9):3206-21. doi: 10.1021/jm101459g. Epub 2011 Apr 12. [21486038 ]
- General Function:
- Monovalent cation:proton antiporter activity
- Specific Function:
- Solute transporter for tetraethylammonium (TEA), 1-methyl-4-phenylpyridinium (MPP), cimetidine, N-methylnicotinamide (NMN), metformin, creatinine, guanidine, procainamide, topotecan, estrone sulfate, acyclovir, ganciclovir and also the zwitterionic cephalosporin, cephalexin and cephradin. Seems to also play a role in the uptake of oxaliplatin (a new platinum anticancer agent). Able to transport paraquat (PQ or N,N-dimethyl-4-4'-bipiridinium); a widely used herbicid. Responsible for the secretion of cationic drugs across the brush border membranes.
- Gene Name:
- SLC47A1
- Uniprot ID:
- Q96FL8
- Molecular Weight:
- 61921.585 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 1.7 uM | Not Available | BindingDB 50001859 |
References
- Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, Morrissey KM, Sali A, Huang Y, Giacomini KM: Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem. 2013 Feb 14;56(3):781-95. doi: 10.1021/jm301302s. Epub 2013 Jan 22. [23241029 ]
- General Function:
- Drug transmembrane transporter activity
- Specific Function:
- Solute transporter for tetraethylammonium (TEA), 1-methyl-4-phenylpyridinium (MPP), cimetidine, N-methylnicotinamide, metformin, creatinine, guanidine, procainamide, topotecan, estrone sulfate, acyclovir, and ganciclovir. Responsible for the secretion of cationic drugs across the brush border membranes.
- Gene Name:
- SLC47A2
- Uniprot ID:
- Q86VL8
- Molecular Weight:
- 65083.915 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 46 uM | Not Available | BindingDB 50001859 |
References
- Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, Morrissey KM, Sali A, Huang Y, Giacomini KM: Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem. 2013 Feb 14;56(3):781-95. doi: 10.1021/jm301302s. Epub 2013 Jan 22. [23241029 ]
- General Function:
- Quaternary ammonium group transmembrane transporter activity
- Specific Function:
- Mediates tubular uptake of organic compounds from circulation. Mediates the influx of agmatine, dopamine, noradrenaline (norepinephrine), serotonin, choline, famotidine, ranitidine, histamin, creatinine, amantadine, memantine, acriflavine, 4-[4-(dimethylamino)-styryl]-N-methylpyridinium ASP, amiloride, metformin, N-1-methylnicotinamide (NMN), tetraethylammonium (TEA), 1-methyl-4-phenylpyridinium (MPP), cimetidine, cisplatin and oxaliplatin. Cisplatin may develop a nephrotoxic action. Transport of creatinine is inhibited by fluoroquinolones such as DX-619 and LVFX. This transporter is a major determinant of the anticancer activity of oxaliplatin and may contribute to antitumor specificity.
- Gene Name:
- SLC22A2
- Uniprot ID:
- O15244
- Molecular Weight:
- 62579.99 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 12 uM | Not Available | BindingDB 50001859 |
References
- Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, Morrissey KM, Sali A, Huang Y, Giacomini KM: Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem. 2013 Feb 14;56(3):781-95. doi: 10.1021/jm301302s. Epub 2013 Jan 22. [23241029 ]