| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:27:47 UTC |
|---|
| Update Date | 2014-12-24 20:25:53 UTC |
|---|
| Accession Number | T3D2908 |
|---|
| Identification |
|---|
| Common Name | Progabide |
|---|
| Class | Small Molecule |
|---|
| Description | Progabide is an analog and prodrug of gamma-aminobutyric acid. It is commonly used in the treatment of epilepsy. It has agonistic activity for both the GABAA and GABAB receptors. Progabide has been investigated for many diseases besides epilepsy, including Parkinson's disease, schizophrenia, clinical depression and anxiety disorder with varying success. |
|---|
| Compound Type | - Amide
- Amine
- Anticonvulsant
- Antidepressant
- Antidepressive Agent
- Antidyskinetic
- Antiparkinson Agent
- Drug
- Ester
- GABA Agonist
- Ketone
- Metabolite
- Organic Compound
- Organochloride
- Organofluoride
- Synthetic Compound
|
|---|
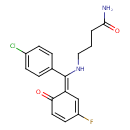
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Gabren | | Gabrene | | Halogabide | | Progabida | | Progabidum |
|
|---|
| Chemical Formula | C17H16ClFN2O2 |
|---|
| Average Molecular Mass | 334.773 g/mol |
|---|
| Monoisotopic Mass | 334.088 g/mol |
|---|
| CAS Registry Number | 62666-20-0 |
|---|
| IUPAC Name | 4-{[(4-chlorophenyl)[(1E)-3-fluoro-6-oxocyclohexa-2,4-dien-1-ylidene]methyl]amino}butanamide |
|---|
| Traditional Name | progabide |
|---|
| SMILES | OC(=N)CCCN\C(C1=CC=C(Cl)C=C1)=C1/C=C(F)C=CC1=O |
|---|
| InChI Identifier | InChI=1S/C17H16ClFN2O2/c18-12-5-3-11(4-6-12)17(21-9-1-2-16(20)23)14-10-13(19)7-8-15(14)22/h3-8,10,21H,1-2,9H2,(H2,20,23)/b17-14+ |
|---|
| InChI Key | InChIKey=DWEQWXSKOHHBNT-SAPNQHFASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as o-quinomethanes. These are organic compounds containing a benzene ring conjugated to a methylidene group and a ketone at carbon atoms 1 and 2, respectively. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | O-quinomethanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - O-quinomethane
- Chlorobenzene
- Halobenzene
- Aryl chloride
- Aryl halide
- Monocyclic benzene moiety
- Benzenoid
- Vinylogous amide
- Carboximidic acid
- Carboximidic acid derivative
- Secondary aliphatic amine
- Enamine
- Fluoroalkene
- Haloalkene
- Secondary amine
- Vinyl fluoride
- Vinyl halide
- Organopnictogen compound
- Organic oxide
- Amine
- Organohalogen compound
- Organochloride
- Organofluoride
- Organonitrogen compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 133-135°C | | Boiling Point | Not Available | | Solubility | 70.9 mg/L | | LogP | 3.06 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000x-5091000000-45493775f8e316fed345 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-1029000000-fc0da8ed87727a709a1f | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-5059000000-63dcad42741a847ae8ff | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000f-9520000000-da113f6ba6c4a6d23a82 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0129000000-3de1ea119383317ba355 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000y-6289000000-b086ccb10b99604720a8 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9330000000-2be76f698dbbc120b6b1 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0009000000-513fcdf8c10f3b29cd13 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f80-0094000000-8c5c8a6b47f57d482e91 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01q9-0090000000-990d46a622dd2ff2264d | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0029000000-8d3aa9ae2fda5750dbf2 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000t-2196000000-d2a509d2c58ae1850d29 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01qj-3490000000-5a245325ccd99decd7c9 | 2021-10-11 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Well absorbed with a bioavailability of 60% |
|---|
| Mechanism of Toxicity | Progabide binds to both GABAA and GABAB receptors located on the terminals of primary afferent fibers. Binding to GABAA results in an increased affinity of the GABA receptor for the amino acid, an augmented flux of chloride ions across the terminal membrane, and an increase in the amount of presynaptic inhibition. Activation of the GABAB receptors retards the influx of calcium ions into the terminals, thereby reducing the evoked release of excitatory amino acids and possibly other transmitters. |
|---|
| Metabolism | Hepatic
Half Life: 4 hours |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Indicated for the treatment of epilepsy. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | May cause a potentially dangerous rash that may develop into Stevens Johnson syndrome, an extremely rare but potentially fatal skin disease. |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00837 |
|---|
| HMDB ID | HMDB14975 |
|---|
| PubChem Compound ID | 5361323 |
|---|
| ChEMBL ID | CHEMBL287631 |
|---|
| ChemSpider ID | 4514729 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Progabide |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Progabide |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - Bartholini G, Scatton B, Zivkovic B, Lloyd KG: GABA receptor agonists and extrapyramidal motor function: therapeutic implications for Parkinson's disease. Adv Neurol. 1987;45:79-83. [3030072 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|