| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:28:00 UTC |
|---|
| Update Date | 2014-12-24 20:25:53 UTC |
|---|
| Accession Number | T3D2937 |
|---|
| Identification |
|---|
| Common Name | Felbamate |
|---|
| Class | Small Molecule |
|---|
| Description | Felbamate is an anticonvulsant drug used in the treatment of epilepsy. It is used to treat partial seizures (with and without generalization) in adults and partial and generalized seizures associated with Lennox-Gastaut syndrome in children. It has a weak inhibitory effect on GABA receptor binding sites. |
|---|
| Compound Type | - Amine
- Anticonvulsant
- Antiepileptic Agent
- Drug
- Ester
- Ether
- Metabolite
- Neuroprotective Agent
- Organic Compound
- Synthetic Compound
|
|---|
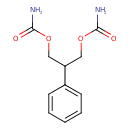
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 2-Phenyl-1,3-propanediol dicarbamate | | Carbamic acid 2-phenyltrimethylene ester | | Carbamic acid 3-carbamoyloxy-2-phenyl-propyl ester | | Felbamato | | Felbamatum | | Felbamic acid | | Felbamyl | | Felbatol | | Taloxa |
|
|---|
| Chemical Formula | C11H14N2O4 |
|---|
| Average Molecular Mass | 238.240 g/mol |
|---|
| Monoisotopic Mass | 238.095 g/mol |
|---|
| CAS Registry Number | 25451-15-4 |
|---|
| IUPAC Name | 3-(carbamoyloxy)-2-phenylpropyl carbamate |
|---|
| Traditional Name | felbamate |
|---|
| SMILES | OC(=N)OCC(COC(O)=N)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C11H14N2O4/c12-10(14)16-6-9(7-17-11(13)15)8-4-2-1-3-5-8/h1-5,9H,6-7H2,(H2,12,14)(H2,13,15) |
|---|
| InChI Key | InChIKey=WKGXYQFOCVYPAC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzene and substituted derivatives. These are aromatic compounds containing one monocyclic ring system consisting of benzene. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Benzene and substituted derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monocyclic benzene moiety
- Carbamic acid ester
- Carbonic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 151.5°C | | Boiling Point | Not Available | | Solubility | Slightly soluble in water | | LogP | 0.3 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fdo-6900000000-0f546bad5ba921f66b40 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-014i-2900000000-880b1fb1822a512a352c | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-016r-1900000000-f04127a0f39b9e0b001f | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-014i-2900000000-880b1fb1822a512a352c | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-016r-1900000000-f04127a0f39b9e0b001f | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-014i-0900000000-edb979350df20b03f995 | 2021-09-20 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-2390000000-02895c4e844f089545e6 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-3920000000-e7c2dfe58df4e1e34dcc | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004l-9500000000-e824587a3374313f7cc4 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-9010000000-b6e0a295dcbb7a9c5266 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-d549b5cb79133b3aa46e | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-6a77d5d551bb1bd0c3a6 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-de230d778fe1524d8977 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0900000000-12a693a2773960f72de0 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014l-4900000000-bf2fec3e77955586472e | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-3900000000-3ae60a2a1bad9c4de09b | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-0ce4d922671bd2797d22 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-94bb7174b97d6eefb992 | 2021-10-11 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0udi-5900000000-673caaab3dded596924c | 2014-09-20 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral. >90% |
|---|
| Mechanism of Toxicity | The mechanism by which felbamate exerts its anticonvulsant activity is unknown, but in animal test systems designed to detect anticonvulsant activity, felbamate has properties in common with other marketed anticonvulsants. In vitro receptor binding studies suggest that felbamate may be an antagonist at the strychnine-insensitive glycine-recognition site of the N-methyl-D-aspartate (NMDA) receptor-ionophore complex. Antagonism of the NMDA receptor glycine binding site may block the effects of the excitatory amino acids and suppress seizure activity. Animal studies indicate that felbamate may increase the seizure threshold and may decrease seizure spread. It is also indicated that felbamate has weak inhibitory effects on GABA-receptor binding, benzodiazepine receptor binding. |
|---|
| Metabolism | Hepatic
Half Life: 20-23 hours |
|---|
| Toxicity Values | LD50: 5000 mg/kg (Oral, Rat) (2) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For use only in those patients who respond inadequately to alternative treatments and whose epilepsy is so severe that a substantial risk of aplastic anemia and/or liver failure is deemed acceptable in light of the benefits conferred by its use. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | May cause a potentially dangerous rash that may develop into Stevens Johnson syndrome, an extremely rare but potentially fatal skin disease. |
|---|
| Symptoms | Not Available |
|---|
| Treatment | General supportive measures should be employed if overdosage occurs. It is not known if felbamate is dialyzable. (4) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00949 |
|---|
| HMDB ID | HMDB15084 |
|---|
| PubChem Compound ID | 3331 |
|---|
| ChEMBL ID | CHEMBL1094 |
|---|
| ChemSpider ID | 3214 |
|---|
| KEGG ID | C07501 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 4995 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Felbamate |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Felbamate |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Link |
|---|
| General References | - Leppik IE, Dreifuss FE, Pledger GW, Graves NM, Santilli N, Drury I, Tsay JY, Jacobs MP, Bertram E, Cereghino JJ, et al.: Felbamate for partial seizures: results of a controlled clinical trial. Neurology. 1991 Nov;41(11):1785-9. [1944909 ]
- Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M: DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008 Jan;36(Database issue):D901-6. Epub 2007 Nov 29. [18048412 ]
- Drugs.com [Link]
- RxList: The Internet Drug Index (2009). [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|