| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-23 18:26:09 UTC |
|---|
| Update Date | 2014-12-24 20:25:57 UTC |
|---|
| Accession Number | T3D3085 |
|---|
| Identification |
|---|
| Common Name | Capsaicin |
|---|
| Class | Small Molecule |
|---|
| Description | Capsaicin is identified as the primary pungent principle in Capsicum fruits. Hot chili peppers that belong to the plant genus Capsicum (family Solanaceae) are among the most heavily consumed spices throughout the world. The capsaicin content of green and red peppers ranges from 0.1 to 1%. Capsaicin evokes numerous biological effects and thus has been the target of extensive., investigations since its initial identification in 1919. One of the most recognized physiological properties of capsaicin is its selective effects on the peripheral part of the sensory nervous system, particularly on the primary afferent neurons. The compound is known to deplete the neurotransmitter of painful impulses known as substance P from the sensory nerve terminals, which provides a rationale for its use as a versatile experimental tool for studying pain mechanisms and also for pharmacotherapy to treat some peripheral painful states, such as rheumatoid arthritis, post-herpetic neuralgia, post-mastectomy pain syndrome and diabetic neuropathy. Considering the frequent consumption of capsaicin as a food additive and its current therapeutic application, correct assessment of any harmful effects of this compound is important from the public health standpoint. Ingestion of large amounts of capsaicin has been reported to cause histopathological and biochemical changes, including erosion of gastric mucosa and hepatic necrosis. However, there are contradictory data on the mutagenicity of capsaicin. A recent epidemiological study conducted in Mexico revealed that consumers of chili pepper were at higher risk for gastric cancer than non-consumers. However, it remains unclear whether capsaicin present in hot chili pepper is a major causative factor in the aetiology of gastric cancer in humans. A growing number of recent studies have focused on anticarcinogenic or antimutagenic phytochemicals, particularly those included in human diet. In summary, capsaicin has dual effects on chemically induced carcinogenesis and mutagenesis. Although a minute amount of capsaicin displays few or no deleterious effects, heavy ingestion of the compound has been associated with necrosis, ulceration and even carcinogenesis. Capsaicin is considered to be metabolized by cytochrome P-450-dependent mixed-function oxidases to reactive species. (1). |
|---|
| Compound Type | - Amide
- Amine
- Ether
- Food Toxin
- Household Toxin
- Lachrymator
- Metabolite
- Natural Compound
- Organic Compound
- Plant Toxin
|
|---|
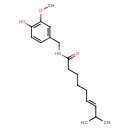
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (E)-8-Methyl-N-vanillyl-6-nonenamide(8cl) | | (E)8-methyl-N-vanillyl-6-Nonenamide | | Axsain | | E-Capsaicin | | epsilon-Capsaicin | | Isodecenoate | | Isodecenoic acid | | Isodecenoic acid vanillylamide | | N-(4-Hydroxy-3-methoxybenzyl)-8-methylnon-trans-6-enamide | | Styptysat | | trans-8-Methyl-N-vanillyl-6-nonenamide | | Transacin | | Zostrix |
|
|---|
| Chemical Formula | C18H27NO3 |
|---|
| Average Molecular Mass | 305.412 g/mol |
|---|
| Monoisotopic Mass | 305.199 g/mol |
|---|
| CAS Registry Number | 404-86-4 |
|---|
| IUPAC Name | (6E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methylnon-6-enamide |
|---|
| Traditional Name | capsaicin |
|---|
| SMILES | [H]\C(CCCCC(O)=NCC1=CC(OC)=C(O)C=C1)=C(\[H])C(C)C |
|---|
| InChI Identifier | InChI=1S/C18H27NO3/c1-14(2)8-6-4-5-7-9-18(21)19-13-15-10-11-16(20)17(12-15)22-3/h6,8,10-12,14,20H,4-5,7,9,13H2,1-3H3,(H,19,21)/b8-6+ |
|---|
| InChI Key | InChIKey=YKPUWZUDDOIDPM-SOFGYWHQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as methoxyphenols. Methoxyphenols are compounds containing a methoxy group attached to the benzene ring of a phenol moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | Methoxyphenols |
|---|
| Direct Parent | Methoxyphenols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Methoxyphenol
- Phenoxy compound
- Anisole
- Methoxybenzene
- Phenol ether
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Fatty acyl
- Fatty amide
- N-acyl-amine
- Carboxamide group
- Secondary carboxylic acid amide
- Carboxylic acid derivative
- Ether
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 65°C | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052o-9860000000-4151cd60d08276e5122a | 2017-07-27 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-03mi-9576000000-fb9f159d71790f546865 | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-014i-0901000000-adba1bb6f254087febe8 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITTOF , negative | splash10-014i-0900000000-5be0e66854b20d7a5a03 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-014i-0900000000-4337ff26dcbd2a77725e | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-014i-0900000000-c94a246143850aa73e44 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-014r-0950000000-3d145ef428e054b3e535 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-014r-0950000000-c3ca097a6480ab798ff3 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-000i-0900000000-4e8f98d1f848f30117d7 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-000i-0900000000-58fab2fdd15893ea71e7 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-000i-0900000000-296a0cb1555da4f1ccd7 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-03di-0209000000-a91e6403d5a0c0b67c2e | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-03di-0209000000-dd0879d3f4f3a8b022c3 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-014i-0149000000-d6a9f6e70ae7cd7aec38 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-014i-0149000000-6fcf63c3bf44a4efc5bd | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-06sl-0075690000-b562b766019cc7cbd98f | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-01x3-0094560000-f354077f4cd51e45b945 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-004i-0002900000-ce1ea9819252343543cd | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-004i-0002911000-6c37470f794c89a66410 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-000i-0900000000-00619747b8012ce6aa64 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-000i-0900000000-3ca73f5212a7643b45d2 | 2017-09-14 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0912000000-95e090cebb8f95d7fe89 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0900000000-6fb8b34936b848190c39 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f79-4900000000-ade1e8ba842adf972c83 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0419000000-80bfd538da9be3f08fe0 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0922000000-00b0b7b3e9df2464d425 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-7900000000-8b112ae03b8d84d338f3 | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (ingestion) (6) ; dermal (6) |
|---|
| Mechanism of Toxicity | The burning and painful sensations associated with capsaicin result from its chemical interaction with sensory neurons. Capsaicin, as a member of the vanilloid family, binds to the vanilloid receptor 1 (VR1). VR1 permits cations to pass through the cell membrane and into the cell when activated. The resulting depolarization of the neuron stimulates it to signal the brain. By binding to the VR1 receptor, the capsaicin molecule produces the same sensation that excessive heat or abrasive damage would cause, explaining why the spiciness of capsaicin is described as a burning sensation. (5) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | LD50: 47200 ug/kg (Oral, Mouse) (4)
LD50: 6500 ug/kg (Intraperitoneal, Mouse) (4)
LD50: 9000 ug/kg (Subcutaneous, Mouse) (4)
LD50: 400 ug/kg (Intravenous, Mouse) (4)
LD50: 7800 ug/kg (Intramuscular, Mouse) (4)
LD50: 1600 ug/kg (Intratracheal, Mouse) (4) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not listed by IARC. |
|---|
| Uses/Sources | Capsaicin is the active component of chili peppers (genus Capsicum). It is a powerful irritant that is commonly used in food products to give them added spice. Capsaicin is also used in topical ointments to relieve the pain of peripheral neuropathy and can be found in pepper spray. (5) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Capsaicin is a powerful irritant and severe over-exposure can result in death. (5) |
|---|
| Symptoms | Capsaicin is a powerful irritant and causes burning or stinging pain to the skin. Ingestion of large amounts can cause nausea, vomiting, abdominal pain and burning diarrhea. Eye exposure produces intense tearing, pain, conjunctivitis and blepharospasm. (5) |
|---|
| Treatment | Capsaicin should be washed off the skin using soap, shampoo, or other detergents, or rubbed off with oily compounds such as vegetable oil, paraffin oil, petroleum jelly, creams, or polyethylene glycol. Burning and pain symptoms can be effectively relieved by cooling from ice, cold water, cold surfaces, or a flow of air. In severe cases, eye burn might be treated symptomatically with topical ophthalmic anaesthetics, while mucous membrane burn can be treated with lidocaine gel. Capsaicin-induced asthma might be treated with nebulized bronchodilators or oral antihistamines or corticosteroids. (5) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB02227 |
|---|
| PubChem Compound ID | 1548943 |
|---|
| ChEMBL ID | CHEMBL294199 |
|---|
| ChemSpider ID | 1265957 |
|---|
| KEGG ID | C06866 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 3374 |
|---|
| BioCyc ID | CAPSAICIN |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Capsaicin |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 244 |
|---|
| Wikipedia Link | Capsaicin |
|---|
| References |
|---|
| Synthesis Reference | Gannett, Peter M.; Nagel, Donald L.; Reilly, Pam J.; Lawson, Terence; Sharpe, Jody; Toth, Bela. Capsaicinoids: their separation, synthesis, and mutagenicity. Journal of Organic Chemistry (1988), 53(5), 1064-71. |
|---|
| MSDS | Link |
|---|
| General References | - Surh YJ, Lee SS: Capsaicin in hot chili pepper: carcinogen, co-carcinogen or anticarcinogen? Food Chem Toxicol. 1996 Mar;34(3):313-6. [8621114 ]
- Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM: Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol. 2007 Aug 15;583(Pt 1):175-93. Epub 2007 Jun 21. [17584831 ]
- Simpson DM, Estanislao L, Brown SJ, Sampson J: An open-label pilot study of high-concentration capsaicin patch in painful HIV neuropathy. J Pain Symptom Manage. 2008 Mar;35(3):299-306. Epub 2007 Oct 23. [17959343 ]
- Lewis RJ Sr. (ed) (2004). Sax's Dangerous Properties of Industrial Materials. 11th Edition. Hoboken, NJ: Wiley-Interscience, Wiley & Sons, Inc.
- Wikipedia. Capsaicin. Last Updated 16 July 2009. [Link]
- Wikipedia. Phytotoxin. Last Updated 7 August 2009. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|