| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-30 17:56:29 UTC |
|---|
| Update Date | 2014-12-24 20:26:00 UTC |
|---|
| Accession Number | T3D3216 |
|---|
| Identification |
|---|
| Common Name | Ethyl acrylate |
|---|
| Class | Small Molecule |
|---|
| Description | Ethyl acrylate is found in pineapple. Ethyl acrylate is a flavouring ingredient Although there are some reports claiming that ethyl acrylate is a carcinogen, major respected bodies regard the evidence of human carcinogenicity as weak and/or inconsistent. The International Agency for Research on Cancer stated, Overall evaluation, Ethyl acrylate is possibly carcinogenic to humans (Group 2B). The United States Environmental Protection Agency (EPA) states, Human studies on occupational exposure to ethyl acrylate… have suggested a relationship between exposure to the chemical(s) and colorectal cancer, but the evidence is conflicting and inconclusive. In a study by the National Toxicology Program (NTP), increased incidences of squamous cell papillomas and carcinomas of the forestomach were observed in rats and mice exposed via gavage (experimentally placing the chemical in the stomach). However, the NTP recently determined that these data were not relevant to human carcinogenicity and removed ethyl acrylate from its list of carcinogens. (Occupational exposure generally involves exposure that occurs regularly, over an extended period of time). Ethyl acrylate is an organic compound primarily used in the preparation of various polymers. It is a clear liquid with an acrid penetrating odor. The human nose is capable of detecting this odor at a thousand times lower concentration then is considered harmful if continuously exposed for some period of time. |
|---|
| Compound Type | - Ester
- Ether
- Food Toxin
- Fragrance Toxin
- Household Toxin
- Industrial/Workplace Toxin
- Metabolite
- Natural Compound
- Organic Compound
- Plant Toxin
- Pollutant
|
|---|
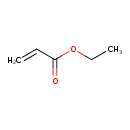
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 2-Propenoic acid ethyl ester | | 2-Propenoic acid, ethyl ester | | 2-Propenoic acid, ethyl ester, homopolymer | | Acrylic acid ethyl ester | | Acrylic acid, ethyl ester | | Acrylic acid, ethyl ester (inhibited) | | Aethylacrylat | | Akrylanem etylu | | Carboset 511 | | CH2=CHCOOC2H5 | | Ethoxycarbonylethylene | | Ethyl 2-propenoate | | Ethyl 2-propenoate, homopolymer | | Ethyl acrylate (inhibited) | | Ethyl acrylate homopolymer | | Ethyl acrylate polymer | | Ethyl acrylate, inhibited | | Ethyl acrylic acid | | Ethyl ester of 2-propenoic acid | | Ethyl propenoate | | Ethyl propenoate, inhibited | | Ethylacrylaat | | Ethylakrylat | | Ethylester kyseliny akrylove | | Etil acrilato | | Etilacrilatului | | Etilacrilatului(roumanian) | | FEMA 2418 | | Poly(acrylic acid, ethyl ester) | | Poly(ethyl acrylate) | | Polyethylacrylate | | Propenoic acid,ethyl ester (ethylacrylate) |
|
|---|
| Chemical Formula | C5H8O2 |
|---|
| Average Molecular Mass | 100.116 g/mol |
|---|
| Monoisotopic Mass | 100.052 g/mol |
|---|
| CAS Registry Number | 140-88-5 |
|---|
| IUPAC Name | ethyl prop-2-enoate |
|---|
| Traditional Name | ethyl acrylate |
|---|
| SMILES | CCOC(=O)C=C |
|---|
| InChI Identifier | InChI=1S/C5H8O2/c1-3-5(6)7-4-2/h3H,1,4H2,2H3 |
|---|
| InChI Key | InChIKey=JIGUQPWFLRLWPJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acrylic acid esters. These are organic compounds containing and ester acrylic acid (CH2=CHC(=O)OH). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Acrylic acids and derivatives |
|---|
| Direct Parent | Acrylic acid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acrylic acid ester
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Clear liquid (7). |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | -71.2°C | | Boiling Point | 99.4°C (210.9°F) | | Solubility | 15 mg/mL at 25°C | | LogP | 1.32 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-9000000000-8762b44567cd7afeb692 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a6r-9000000000-25b2b71f04040f0959dd | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-9000000000-9fb2ce6a8c6e827cca22 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-9000000000-96cab8193fbae26c0e12 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-9000000000-8762b44567cd7afeb692 | 2018-05-18 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a6r-9000000000-25b2b71f04040f0959dd | 2018-05-18 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-9000000000-9fb2ce6a8c6e827cca22 | 2018-05-18 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-9000000000-96cab8193fbae26c0e12 | 2018-05-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a6r-9000000000-b44a167f27a11982ce88 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f6t-9000000000-b878ab9b418ac14be652 | 2015-04-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udj-9000000000-75144951146aefa9cb6c | 2015-04-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-9000000000-e6c22ca53e8d3d79f0b3 | 2015-04-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-9000000000-6636b98068f5d8e94960 | 2021-09-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fk9-9000000000-e0f16a81ce309da5b9a7 | 2021-09-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9000000000-e973a9ae833442ead826 | 2021-09-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-6900000000-afe7a2cd2b3f23523df8 | 2015-04-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9200000000-b2987d0fef61f6de593e | 2015-04-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-63a2abd18e012c3564b2 | 2015-04-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-9000000000-47264722dae793143c2b | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4r-9000000000-7bd873751816c51e989f | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-55bfb6f6731ad493ad1d | 2021-09-23 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0a4i-9000000000-200bb2f29d9c1c5820c7 | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 15.09 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Inhalation (7) ; oral (7) ; dermal (7) ; eye contact (7). |
|---|
| Mechanism of Toxicity | Ethyl acrylate causes gastric lesions depending upon upon the rate of chemical delivery to stomach tissue as a result of interaction of the parent molecule or metabolites (other than hydrolysis products), with subcellular sites in stomach tissue (2). |
|---|
| Metabolism | Ethyl acrylate is hydrolized to acrylic acid in liver, kidney, and lung homogenates. It is suggested to bind to glutathione spontaneaoulsy and after catalysis by liver glutathione-S-transferase. The major metabolites of ethyl acrylate are mercapturic acids od ethyl acrylate and acrylic acid. Three metabolites were identified in a study: 3-hydroxypropionic acid and two mercapturic acids. N-Acetyl-S-(2-carboxyethyl)cysteine arises by glutathione conjugation of acrylic acid, while N-acetyl-S-(2-carboxyethyl)cysteine ethyl ester derives from the conjugation of intact ethyl acrylate.(1, 3, 4). |
|---|
| Toxicity Values | LD50: 760-1020 mg/kg (Oral, Rat) (8)
LC50: 1000-2000 ppm over 4 hours (Inhalation, Rat) (6) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 2B, possibly carcinogenic to humans. (14) |
|---|
| Uses/Sources | Ethyl acrylate is used in the production of polymers including resins, plastics, rubber, and denture material (15). |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Thickened forestomach mucosa, forestomach inflammation and lesions, and abdominal adhesions; swelling of renal tubules and the liver, minor lesions on the liver and lung, and increased kidney weight may result from poisoning (17). |
|---|
| Symptoms | Lethargy and convulsions may occur if vapors are inhaled in high concentrations. Ethyl acrylate is irritating to the mucous membranes of the gastrointestinal tract and respiratory system. Prolonged exposure may produce drowsiness, headache and nausea . Iching of the skin and the face can also occur (9, 7). |
|---|
| Treatment | For eye contamination, flush eyes immediately with water. Irrigate each eye continuously with normal saline during transport. Do not use emetics. For ingestion, rinse mouth and administer 5 ml/kg up to 200 ml of water for dilution if the patient can swallow, has a strong gag reflex, and does not drool. Administer activated charcoal. (5) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB33978 |
|---|
| PubChem Compound ID | 8821 |
|---|
| ChEMBL ID | CHEMBL52084 |
|---|
| ChemSpider ID | 8490 |
|---|
| KEGG ID | C19238 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | CPD-8095 |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Ethyl acrylate |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 593 |
|---|
| Wikipedia Link | Ethyl_acrylate |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Link |
|---|
| General References | - Miller RR, Ayres JA, Rampy LW, McKenna MJ: Metabolism of acrylate esters in rat tissue homogenates. Fundam Appl Toxicol. 1981 Nov-Dec;1(6):410-4. [7185591 ]
- Ghanayem BI, Maronpot RR, Matthews HB: Ethyl acrylate-induced gastric toxicity. II. Structure-toxicity relationships and mechanism. Toxicol Appl Pharmacol. 1985 Sep 15;80(2):336-44. [4024123 ]
- Inoue K: [Analysis and its application for prevention of side-effects of drugs and for evaluation of drug responsiveness]. Yakugaku Zasshi. 2004 Jun;124(6):293-9. [15170064 ]
- Klaassen A, Glykys J, Maguire J, Labarca C, Mody I, Boulter J: Seizures and enhanced cortical GABAergic inhibition in two mouse models of human autosomal dominant nocturnal frontal lobe epilepsy. Proc Natl Acad Sci U S A. 2006 Dec 12;103(50):19152-7. Epub 2006 Dec 4. [17146052 ]
- Gidlof AC, Ocaya P, Olofsson PS, Torma H, Sirsjo A: Differences in retinol metabolism and proliferative response between neointimal and medial smooth muscle cells. J Vasc Res. 2006;43(4):392-8. Epub 2006 Jul 6. [16837774 ]
- American Conference of Governmental Industrial Hygienists, Inc. (1991). Documentation of the Threshold Limit Values and Biological Exposure Indices. 6th ed. Volumes I, II, III. Cincinnati, OH: American Conference of Governmental Industrial Hygienists.
- Clayton GD and Clayton FE (eds) (1993-1994). Patty's Industrial Hygiene and Toxicology. Volumes 2A, 2B, 2C, 2D, 2E, 2F: Toxicology. 4th ed. New York, NY: John Wiley & Sons Inc.
- Kirk-Othmer Encyclopedia of Chemical Technology (1978-1984). 3rd ed. Volumes 1-26. New York, NY: John Wiley and Sons.
- The Merck Index (1983). The Merck Index. 10th ed. Rahway, New Jersey: Merck Co., Inc.
- Bronstein, AC, Currance PL (1994). Emergency Care for Hazardous Materials Exposure. 2nd ed. St. Louis, MO. Mosby Lifeline. 1994.
- IARC (1986). Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-Present. (Multivolume work).
- National Toxicology Program (1986). Fiscal Year 1986 Annual Plan.
- Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
- Quantyka (2008). Safety Data Sheet for 4-Allylanisole. [Link]
- Wikipedia. Ethyl Acrylate. Last Updated 20 July 2009. [Link]
- Technology Transfer Network, Air Toxics Web Site (2009). Ethyl Acrylate. [Link]
- IARC (1986). Monograph of Ethyl Acrylate. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|