| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-30 17:58:33 UTC |
|---|
| Update Date | 2014-12-24 20:26:06 UTC |
|---|
| Accession Number | T3D3469 |
|---|
| Identification |
|---|
| Common Name | Polyvinyl acetate |
|---|
| Class | Small Molecule |
|---|
| Description | Polyvinyl acetate is a rubbery synthetic polymer. It is a component of glue and is used mainly as an adhesive for porous materials, particularly for wood, paper, and cloth. While polyvinyl acetate itself is not considered hazardous, it usually contains trace amounts of its precursor, vinyl acetate, which is toxic. (3, 4) |
|---|
| Compound Type | - Ester
- Ether
- Food Toxin
- Glue Component
- Household Toxin
- Industrial/Workplace Toxin
- Lachrymator
- Organic Compound
- Synthetic Compound
|
|---|
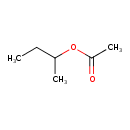
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1-acetoxyethylene | | Acetate de vinyle | | Acetic acid ethenyl ester | | Acetic acid ethylene ether | | Acetic acid vinyl ester | | Acetoxyethene | | Acetoxyethylene | | Essigsaeurevinylester | | Ethanoic acid, ethenyl ester | | Ethenyl ethanoate | | Octan winylu | | Polyvinyl acetic acid | | Vinile (acetato di) | | Vinyl a monomer | | Vinyl ethanoate | | Vinylester kyseliny octove | | VYAC | | Zeset t |
|
|---|
| Chemical Formula | C6H12O2 |
|---|
| Average Molecular Mass | 116.158 g/mol |
|---|
| Monoisotopic Mass | 116.084 g/mol |
|---|
| CAS Registry Number | 9003-20-7 |
|---|
| IUPAC Name | butan-2-yl acetate |
|---|
| Traditional Name | sec-butyl acetate |
|---|
| SMILES | CCC(C)OC(C)=O |
|---|
| InChI Identifier | InChI=1/C6H12O2/c1-4-5(2)8-6(3)7/h5H,4H2,1-3H3 |
|---|
| InChI Key | InChIKey=DCKVNWZUADLDEH-UHFFFAOYNA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as carboxylic acid esters. These are carboxylic acid derivatives in which the carbon atom from the carbonyl group is attached to an alkyl or an aryl moiety through an oxygen atom (forming an ester group). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Carboxylic acid derivatives |
|---|
| Direct Parent | Carboxylic acid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Actin Filament
- Apical Membrane
- Basolateral Membrane
- Cell junction
- Cell surface
- Cytoplasm
- Cytoskeleton
- Cytosol
- Endocytic Vesicle
- Endosome
- Extracellular
- Extracellular matrix
- Golgi apparatus
- Intermediate Filament
- Lysosome
- Membrane Fraction
- Microtubule
- Mitochondrion
- Nuclear Membrane
- Nucleoplasm
- Peroxisome
- Plasma Membrane
- Ribosome
- Sarcoplasmic Reticulum
- Soluble Fraction
- Synaptic Vesicle
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Apoptosis | Not Available | map04210 | | Phagosome | Not Available | map04145 | | Long-term potentiation | Not Available | map04720 | | Endocytosis | Not Available | map04144 | | Spliceosome | Not Available | map03040 | | Proteasome | Not Available | Not Available | | Phenothiazines | Not Available | Not Available | | Melanogenesis | Not Available | map04916 | | Aflatoxin Biosynthesis | Not Available | Not Available |
|
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a6u-9000000000-70f0500e0a8ad185a938 | 2021-09-23 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-066r-9700000000-6d7bd6285e09a53c948c | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9000000000-2d4732fb6d47bddfaccf | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-4c4b084a298fbbc1ec68 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01b9-9700000000-c162bd3420dddd9efebf | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ab9-9100000000-7400fa5853622667071a | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-f9199dc19134dc680705 | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (5) ; Inhalation (5) ; Dermal (5) |
|---|
| Mechanism of Toxicity | Polyvinyl acetate usually contains trace amounts of its precursor, vinyl acetate. One of the metabolites of vinyl acetate, acetaldehyde, is a known animal carcinogen. Acetaldehyde can form adducts with DNA, causing damage such as cross-links. (5, 1) |
|---|
| Metabolism | Vinyl acetate may be absorbed following ingestion, inhalation, or dermal exposure, and distributes throughout the body. It is rapidly hydrolyzed by esterases in the blood to acetate and the unstable intermediate, vinyl alcohol. Vinyl alcohol is then rapidly converted to acetaldehyde, which in turn is metabolized to acetate in the liver. This in turn is incorporated into the "2 carbon pool" of normal body metabolism and eventually forms carbon dioxide as the major breakdown product, which is expired. (5) |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 3, not classifiable as to its carcinogenicity to humans. (2) |
|---|
| Uses/Sources | Polyvinyl acetate is a component of glue and is used mainly as an adhesive for porous materials, particularly for wood, paper, and cloth. (3) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Vinyl acetate may affect the immune system. It may also be a carcinogen. (5) |
|---|
| Symptoms | Inhalation of vinyl acetate irritates the eyes, nose, and throat. Skin contact causes irritation and blisters. (5) |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 7904 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 7472 |
|---|
| KEGG ID | C12282 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Polyvinyl acetate |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 16103 |
|---|
| Wikipedia Link | Polyvinyl_acetate |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D3469.pdf |
|---|
| General References | - Brooks PJ, Theruvathu JA: DNA adducts from acetaldehyde: implications for alcohol-related carcinogenesis. Alcohol. 2005 Apr;35(3):187-93. [16054980 ]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
- Wikipedia. Polyvinyl acetate. Last Updated 21 July 2009. [Link]
- Wikipedia. Vinyl acetate. Last Updated 26 July 2009. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (1999). Toxicological profile for vinyl acetate. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|