| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-11-28 02:08:54 UTC |
|---|
| Update Date | 2014-12-24 20:26:15 UTC |
|---|
| Accession Number | T3D3629 |
|---|
| Identification |
|---|
| Common Name | Sodium lauryl sulfate |
|---|
| Class | Small Molecule |
|---|
| Description | Sodium lauryl sulfate (SLS) is an anionic surfactant used in many cleaning and hygiene products. It is naturally derived from coconut and/or palm kernel oil. It usually consisting of a mixture of sodium alkyl sulfates, mainly the lauryl. SLS lowers surface tension of aqueous solutions and is used as fat emulsifier, wetting agent, and detergent in cosmetics, pharmaceuticals and toothpastes. It is also used in creams and pastes to properly disperse the ingredients and as research tool in protein biochemistry. SLS also has some microbicidal activity. The molecule has a tail of 12 carbon atoms, attached to a sulfate group, giving the molecule the amphiphilic properties required of a detergent. SLS is a highly effective surfactant used in any task requiring the removal of oily stains and residues. As such the compound is found in high concentrations in industrial products including engine degreasers, floor cleaners, and car wash soaps. In household products, SLS is used in lower concentrations with toothpastes, shampoos, and shaving foams. It is an important component in bubble bath formulations for its thickening effect and its ability to create a lather. SLS may irritate the skin and eyes. (13) |
|---|
| Compound Type | - Cosmetic Toxin
- Drug
- Ester
- Food Toxin
- Household Toxin
- Industrial/Workplace Toxin
- Lachrymator
- Organic Compound
- Plant Toxin
- Surface-Active Agent
- Synthetic Compound
|
|---|
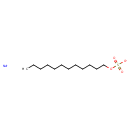
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Anticerumen | | Emal 10 | | Empicol | | Genapol LSS | | Irium | | Laurylsiran sodny | | NaDS | | Natrium laurylsulfuricum | | SDS | | SLS | | Sodium dodecyl sulfate | | Sodium dodecyl sulphate | | Sodium dodecylsulfate | | Sodium laurilsulfate | | Sodium lauryl sulfic acid | | Sodium lauryl sulphate | | Sodium lauryl sulphic acid |
|
|---|
| Chemical Formula | C12H25NaO4S |
|---|
| Average Molecular Mass | 288.379 g/mol |
|---|
| Monoisotopic Mass | 288.137 g/mol |
|---|
| CAS Registry Number | 151-21-3 |
|---|
| IUPAC Name | sodium dodecyl sulfate |
|---|
| Traditional Name | sodium dodecyl sulfate |
|---|
| SMILES | [Na+].CCCCCCCCCCCCOS([O-])(=O)=O |
|---|
| InChI Identifier | InChI=1S/C12H26O4S.Na/c1-2-3-4-5-6-7-8-9-10-11-12-16-17(13,14)15;/h2-12H2,1H3,(H,13,14,15);/q;+1/p-1 |
|---|
| InChI Key | InChIKey=DBMJMQXJHONAFJ-UHFFFAOYSA-M |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sulfuric acid monoesters. These are organic compounds containing the sulfuric acid monoester functional group, with the generic structure ROS(O)(=O)=O, (R=organyl group). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic sulfuric acids and derivatives |
|---|
| Sub Class | Sulfuric acid esters |
|---|
| Direct Parent | Sulfuric acid monoesters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkyl sulfate
- Sulfate-ester
- Sulfuric acid monoester
- Organic alkali metal salt
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organic sodium salt
- Organic salt
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 205.5°C | | Boiling Point | Not Available | | Solubility | 1E+005 mg/L | | LogP | 1.6 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-070g-7930000000-112a35183d2ba1b6ef5c | 2017-08-28 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-000i-0090000000-12b55f58de196159b535 | 2012-07-25 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-00di-9000000000-55a0d8975342d4745eee | 2012-07-25 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-00di-9000000000-55a0d8975342d4745eee | 2012-07-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1940000000-94b9e506e5597ccf8b74 | 2019-02-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014l-4900000000-1642b024e5c3071571fd | 2019-02-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052g-9700000000-4438a3c78dc9e50e9116 | 2019-02-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-c3eba39e0193d936fd32 | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0019-7390000000-033056a3eb10f6c1a5ba | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9100000000-e35f9421417f46956df2 | 2019-02-23 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, DMSO-d6, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 22.53 MHz, DMSO-d6, experimental) | Not Available | 2014-09-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (3) ; inhalation (3) ; dermal (11) |
|---|
| Mechanism of Toxicity | While sodium lauryl sulfate itself is not toxic, it is a nitrosating agent. Nitrosating agents may decompose and/or react to cause nitrosamine contamination. Nitrosamines are produced from secondary amines and amides in the presence of nitrite ions and are believed to be carcinogenic. Once in the body, nitrosamines are activated by cytochrome P-450 enzymes. They are then believed to induce their carcinogenic effects by forming DNA adducts at the N- and O-atoms. (11, 12, 1, 2, 3) |
|---|
| Metabolism | Nitrosamines can enter the body via ingestion, inhalation, or dermal contact. Once in the body, nitrosamines are metabolized by cytochrome P-450 enzymes, which essentially activates them into carcinogens. (1, 2) |
|---|
| Toxicity Values | LD50: 1288 mg/kg (Oral, Rat) (10)
LD50: 210 mg/kg (Intraperitoneal, Rat) (10)
LD50: 118 mg/kg (Intravenous, Rat) (10) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not listed by IARC. Certain nitrosamines are classified by IARC as either probably or possibly carcinogenic to humans (Groups 2A and 2B, respectively). (14) |
|---|
| Uses/Sources | SLS is a highly effective surfactant used in any task requiring the removal of oily stains and residues. As such the compound is found in high concentrations in industrial products including engine degreasers, floor cleaners, and car wash soaps. In household products, SLS is used in lower concentrations with toothpastes, shampoos, and shaving foams. It is an important component in bubble bath formulations for its thickening effect and its ability to create a lather. SLS may irritate the skin and eyes. (13) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | SLS can cause skin and eye irritation, as well as canker sores when used in toothpasete. SLS may also react to produce nitrosamines, which are believed to be carcinogenic. (12, 13) |
|---|
| Symptoms | SLS can cause skin and eye irritation, as well as canker sores when used in toothpasete. (13) |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00815 |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 9028 |
|---|
| ChEMBL ID | CHEMBL23393 |
|---|
| ChemSpider ID | Not Available |
|---|
| KEGG ID | C11166 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 8984 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Sodium_lauryl_sulfate |
|---|
| References |
|---|
| Synthesis Reference | Willi Breitzke, Hermann Hensen, “Aqueous preparations of sodium lauryl sulfate and myristyl sulfate having a low cloud point useful in making toothpastes.” U.S. Patent US4876035, issued June, 1960. |
|---|
| MSDS | T3D3629.pdf |
|---|
| General References | - Oyama T, Sugio K, Uramoto H, Iwata T, Onitsuka T, Isse T, Nozoe T, Kagawa N, Yasumoto K, Kawamoto T: Increased cytochrome P450 and aryl hydrocarbon receptor in bronchial epithelium of heavy smokers with non-small cell lung carcinoma carries a poor prognosis. Front Biosci. 2007 May 1;12:4497-503. [17485391 ]
- Sasaki S, Sata F, Katoh S, Saijo Y, Nakajima S, Washino N, Konishi K, Ban S, Ishizuka M, Kishi R: Adverse birth outcomes associated with maternal smoking and polymorphisms in the N-Nitrosamine-metabolizing enzyme genes NQO1 and CYP2E1. Am J Epidemiol. 2008 Mar 15;167(6):719-26. doi: 10.1093/aje/kwm360. Epub 2008 Jan 23. [18218609 ]
- Drablos F, Feyzi E, Aas PA, Vaagbo CB, Kavli B, Bratlie MS, Pena-Diaz J, Otterlei M, Slupphaug G, Krokan HE: Alkylation damage in DNA and RNA--repair mechanisms and medical significance. DNA Repair (Amst). 2004 Nov 2;3(11):1389-407. [15380096 ]
- Agner T: Susceptibility of atopic dermatitis patients to irritant dermatitis caused by sodium lauryl sulphate. Acta Derm Venereol. 1991;71(4):296-300. [1681644 ]
- Marrakchi S, Maibach HI: Sodium lauryl sulfate-induced irritation in the human face: regional and age-related differences. Skin Pharmacol Physiol. 2006;19(3):177-80. Epub 2006 May 4. [16679819 ]
- Loffler H, Effendy I: Skin susceptibility of atopic individuals. Contact Dermatitis. 1999 May;40(5):239-42. [10344477 ]
- Chahine L, Sempson N, Wagoner C: The effect of sodium lauryl sulfate on recurrent aphthous ulcers: a clinical study. Compend Contin Educ Dent. 1997 Dec;18(12):1238-40. [9656847 ]
- Herlofson BB, Barkvoll P: The effect of two toothpaste detergents on the frequency of recurrent aphthous ulcers. Acta Odontol Scand. 1996 Jun;54(3):150-3. [8811135 ]
- Piret J, Desormeaux A, Bergeron MG: Sodium lauryl sulfate, a microbicide effective against enveloped and nonenveloped viruses. Curr Drug Targets. 2002 Feb;3(1):17-30. [11899262 ]
- Lewis RJ (1996). Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold.
- Wikipedia. Nitrosamine. Last Updated 16 November 2009. [Link]
- Organic Natural Health (1998). Cancer Causing Toxic Chemical Ingredients in Cosmetic and Skin Care Products. [Link]
- Wikipedia. Sodium lauryl sulfate. Last Updated 12 November 2009. [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|