| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2010-04-21 19:31:18 UTC |
|---|
| Update Date | 2014-12-24 20:26:22 UTC |
|---|
| Accession Number | T3D3686 |
|---|
| Identification |
|---|
| Common Name | Methylergonovine |
|---|
| Class | Small Molecule |
|---|
| Description | Methylergonovine is only found in individuals that have used or taken this drug. It is a homolog of ergonovine containing one more CH2 group (Merck Index, 11th ed). Methylergonovine acts directly on the smooth muscle of the uterus and increases the tone, rate, and amplitude of rhythmic contractions through binding and the resultant antagonism of the dopamine D1 receptor. Thus, it induces a rapid and sustained tetanic uterotonic effect which shortens the third stage of labor and reduces blood loss. |
|---|
| Compound Type | - Amide
- Amine
- Drug
- Metabolite
- Organic Compound
- Oxytocic
- Serotonin Antagonist
- Synthetic Compound
- Vasoconstrictor Agent
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 9,10-Didehydro-N-[1-(hydroxymethyl)-propyl]-D-lysergamide | | Basofortina | | Bledstop | | D-lysergic acid 1-butanolamide | | Demergin | | Ergogin | | Ergomed | | Ergomin | | Ergotyl | | Expogin | | Glomethyl | | Ingagen-M | | Mem | | Mergot | | Mergotrex | | Metenarin | | Metermin | | Méthergin | | Methergine | | Metherinal | | Metherspan | | Methovin | | Methylergobasin | | Methylergobasine | | Methylergobrevin | | Methylergometrin | | Methylergometrine | | Methylergometrinum | | Methylergonovin | | Methylergonovine maleate | | Metilat | | Metiler | | Metilergometrina | | Metrine | | Metrol | | Metvell | | Myomergin | | Myometril | | Neo-ergo | | Partan M | | Pospargin | | Satergin | | Usamema | | Utergin | | Uterine | | Uterjin | | Uterowin | | Utesel |
|
|---|
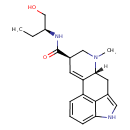
| Chemical Formula | C20H25N3O2 |
|---|
| Average Molecular Mass | 339.431 g/mol |
|---|
| Monoisotopic Mass | 339.195 g/mol |
|---|
| CAS Registry Number | 113-42-8 |
|---|
| IUPAC Name | (4R,7R)-N-[(2S)-1-hydroxybutan-2-yl]-6-methyl-6,11-diazatetracyclo[7.6.1.0²,⁷.0¹²,¹⁶]hexadeca-1(16),2,9,12,14-pentaene-4-carboxamide |
|---|
| Traditional Name | methylergonovine |
|---|
| SMILES | [H][C@](CC)(CO)N=C(O)[C@@]1([H])CN(C)[C@]2([H])CC3=CNC4=CC=CC(=C34)C2=C1 |
|---|
| InChI Identifier | InChI=1S/C20H25N3O2/c1-3-14(11-24)22-20(25)13-7-16-15-5-4-6-17-19(15)12(9-21-17)8-18(16)23(2)10-13/h4-7,9,13-14,18,21,24H,3,8,10-11H2,1-2H3,(H,22,25)/t13-,14+,18-/m1/s1 |
|---|
| InChI Key | InChIKey=UNBRKDKAWYKMIV-QWQRMKEZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as lysergamides. These are amides of Lysergic acids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Ergoline and derivatives |
|---|
| Sub Class | Lysergic acids and derivatives |
|---|
| Direct Parent | Lysergamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Lysergic acid amide
- Indoloquinoline
- Benzoquinoline
- Pyrroloquinoline
- Quinoline-3-carboxamide
- Quinoline
- 3-alkylindole
- Indole
- Indole or derivatives
- Isoindole or derivatives
- Aralkylamine
- Benzenoid
- Heteroaromatic compound
- Pyrrole
- Tertiary aliphatic amine
- Tertiary amine
- Secondary carboxylic acid amide
- Carboxamide group
- Amino acid or derivatives
- Azacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Amine
- Alcohol
- Organonitrogen compound
- Organooxygen compound
- Carbonyl group
- Organic nitrogen compound
- Primary alcohol
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 172°C | | Boiling Point | Not Available | | Solubility | 25 mg/mL | | LogP | 1.2 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-3914000000-d6349f005e3253821421 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-01ba-4095000000-07cac91c483c45e0b4fc | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-006x-1019000000-5743eb114639a8b4cd68 | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uk9-4093000000-2f215482a3bef9973b4f | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-3940000000-bf7ef321bb426974d49b | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0019000000-25ad156f1bdbadfdb37f | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05g0-2169000000-04eae81fd7d7bb4dbf72 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fu-8290000000-0f17abb269c859d8cdac | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0019000000-011f8e74477076271412 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006x-1069000000-38039c479f71c2219d4d | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-0190000000-68a0ea065cbe0f2aeef0 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-7d83f1b1716bd17dabc8 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0009000000-4856ff7ce1f702c342f8 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0avl-5290000000-bb401d09f7282c1260d2 | 2021-09-22 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Ingestion (6). Absorption is rapid after oral (60% bioavailability) and intramuscular (78% bioavailability) administration. |
|---|
| Mechanism of Toxicity | Ergoline alkaloids have been shown to have the significant affinity towards the 5-HT1 and 5-HT2 serotonin receptors, D1 and D2 dopamine receptors, and alpha-adrenergic receptors. This can result in a number of different effects, including vasoconstriction, convulsions, and hallucinations. (2, 3, 4) |
|---|
| Metabolism | Hepatic, with extensive first-pass metabolism.
Route of Elimination: Ergot alkaloids are mostly eliminated by hepatic metabolism and excretion, and the decrease in bioavailability following oral administration is probably a result of first-pass metabolism in the liver.
Half Life: 3.39 hours |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Methylergometrine is a synthetic analogue of ergonovine, an alkaloid of the ergoline family that occurs in various species of vines of the Convolvulaceae (morning glory) family and in some species of lower fungi like other ergoline alkaloids. Methylergometrine is a blood vessel constrictor and smooth muscle agonist most commonly used to prevent or control excessive bleeding following childbirth and spontaneous or elective abortion. It also causes uterine contractions to aid in expulsion of retained products of conception after a missed abortion (miscarriage in which all or part of the fetus remains in the uterus) and to help deliver the placenta after childbirth. The drug is currently prepared via reaction of (+)-lysergic acid with L-(+)-aminobutanol, using different coupling reagents, and is available under the name Methergine as tablets or injection (IM or IV) or in liquid form to be taken orally. (5, 8) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Ingestion of ergoline alkaloids is known to cause the disease ergotism. Ergotism occurs in two forms, gangrenous and convulsive, likely depending on the different kinds and amounts of ergoline alkaloids present. (1) |
|---|
| Symptoms | Convulsive ergotism can cause painful seizures and spasms, diarrhea, paresthesias, itching, headaches, nausea and vomiting. Usually the gastrointestinal effects precede the central nervous system effects. As well as seizures there can be hallucinations and mental effects including mania or psychosis. Gangrenous ergotism causes dry gangrene as a result of vasoconstriction induced in the more poorly vascularized distal structures, such as the fingers and toes. Symptoms include desquamation, weak periphery pulse, loss of peripheral sensation, edema and ultimately the death and loss of affected tissues. (6) |
|---|
| Treatment | Treatment for ergotism consists of vasodilators, anticoagulants and low molecular weight dextrans. If necessary, a sympathetic nerve blockade may be carried out, such as brachial plexus blockade. Temporary sedation (e.g. haloperidol) will be necessary in hallucination and diazepam is used for convulsions. There is no specific antidote. (7) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00353 |
|---|
| HMDB ID | HMDB14497 |
|---|
| PubChem Compound ID | 8226 |
|---|
| ChEMBL ID | CHEMBL1201356 |
|---|
| ChemSpider ID | 7933 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 775307 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Methylergonovine |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Link |
|---|
| General References | - Richard JL: Some major mycotoxins and their mycotoxicoses--an overview. Int J Food Microbiol. 2007 Oct 20;119(1-2):3-10. Epub 2007 Jul 31. [17719115 ]
- Mantegani S, Brambilla E, Varasi M: Ergoline derivatives: receptor affinity and selectivity. Farmaco. 1999 May 30;54(5):288-96. [10418123 ]
- Schiff PL: Ergot and its alkaloids. Am J Pharm Educ. 2006 Oct 15;70(5):98. [17149427 ]
- Kvernmo T, Hartter S, Burger E: A review of the receptor-binding and pharmacokinetic properties of dopamine agonists. Clin Ther. 2006 Aug;28(8):1065-78. [16982285 ]
- Wikipedia. Ergoline. Last Updated 2 April 2010. [Link]
- Wikipedia. Ergotism. Last Updated 6 April 2010. [Link]
- Van den Enden, E. (2004). Illustrated Lecture Notes on Tropical Medicine. [Link]
- Wikipedia. Methylergometrine. Last Updated 13 April 2010. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|