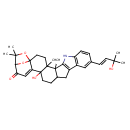
Paspalitrem B (T3D3778)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2010-06-10 15:41:04 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:26:32 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D3778 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Paspalitrem B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Paspalitrem B is a tremorgenic mycotoxin that has been found in fungi of the genera Penicillium and Aspergillus. It may be found in contaminated cereal crops such as oats, barley, millet, corn and rice. Tremorgenic mycotoxins affect central nervous system activity and have been implicated in a number of neurologic diseases of cattle collectively known as "staggers syndromes". (1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C32H39NO5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 517.656 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 517.283 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 63764-58-9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | 19-hydroxy-11-[(1E)-3-hydroxy-3-methylbut-1-en-1-yl]-4,5,24,24-tetramethyl-25,26-dioxa-7-azaheptacyclo[21.2.1.0¹,²⁰.0⁴,¹⁹.0⁵,¹⁶.0⁶,¹⁴.0⁸,¹³]hexacosa-6(14),8(13),9,11,20-pentaen-22-one | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | 19-hydroxy-11-[(1E)-3-hydroxy-3-methylbut-1-en-1-yl]-4,5,24,24-tetramethyl-25,26-dioxa-7-azaheptacyclo[21.2.1.0¹,²⁰.0⁴,¹⁹.0⁵,¹⁶.0⁶,¹⁴.0⁸,¹³]hexacosa-6(14),8(13),9,11,20-pentaen-22-one | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | CC(C)(O)\C=C\C1=CC2=C(NC3=C2CC2CCC4(O)C5=CC(=O)C6OC5(CCC4(C)C32C)OC6(C)C)C=C1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C32H39NO5/c1-27(2,35)11-9-18-7-8-22-20(15-18)21-16-19-10-12-31(36)24-17-23(34)26-28(3,4)38-32(24,37-26)14-13-29(31,5)30(19,6)25(21)33-22/h7-9,11,15,17,19,26,33,35-36H,10,12-14,16H2,1-6H3/b11-9+ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=BZJWARDOYGJZHQ-PKNBQFBNSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as naphthopyrans. Naphthopyrans are compounds containing a pyran ring fused to a naphthalene moiety. Furan is a 6 membered-ring non-aromatic ring with five carbon and one oxygen atoms. Naphthalene is a polycyclic aromatic hydrocarbon made up of two fused benzene rings. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Naphthopyrans | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Naphthopyrans | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | White powder. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Oral, dermal, inhalation, and parenteral (contaminated drugs). (3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Tremorgenic mycotoxins exert their toxic effects by interfering with neurotransmitter release, possibly by causing degeneration of nerve terminals. They are thought to inhibit gamma-aminobutyric acid (GABA) receptors, both pre- and postsynaptic, as well as inhibit transmitter breakdown at the GABA-T receptors. This would initially increase neurotransmitter levels, potentiating the GABA-induced chloride current, then lead to decreased levels of neurotransmitter in the synapse. In addition, paspalitrem B inhibits presynaptic high-conductance Ca+2 activated maxi-K+ channels in the smooth muscle. (1, 2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | Paspalitrem B is a tremorgenic mycotoxin that has been found in fungi of the genera Penicillium and Aspergillus. It may be found in contaminated cereal crops such as oats, barley, millet, corn and rice. (1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Tremorgenic mycotoxins affect central nervous system activity. They cause a neurological disease of cattle known as "staggers syndrome". (1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Tremorgenic mycotoxins affect central nervous system activity, inducing neurologic symptoms including mental confusion, paralysis, tremors, seizures, and death. They cause a neurological disease of cattle known as "staggers syndrome", which is characterized by muscle tremors, hyperexcitability, convulsions and ataxia. (1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | To control severe tremors caused by tremorgenic mycotoxins, methocarbamol should be administered. Generalized seizures may be treated with diazepam followed by methocarbamol or a barbiturate such as pentobarbital sodium. Gastric lavage should be performed and activated charcoal administered to limit further absorption of toxins. (4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Succinate-semialdehyde dehydrogenase binding
- Specific Function:
- Catalyzes the conversion of gamma-aminobutyrate and L-beta-aminoisobutyrate to succinate semialdehyde and methylmalonate semialdehyde, respectively. Can also convert delta-aminovalerate and beta-alanine.
- Gene Name:
- ABAT
- Uniprot ID:
- P80404
- Molecular Weight:
- 56438.405 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- Voltage-gated potassium channel activity
- Specific Function:
- Potassium channel activated by both membrane depolarization or increase in cytosolic Ca(2+) that mediates export of K(+). It is also activated by the concentration of cytosolic Mg(2+). Its activation dampens the excitatory events that elevate the cytosolic Ca(2+) concentration and/or depolarize the cell membrane. It therefore contributes to repolarization of the membrane potential. Plays a key role in controlling excitability in a number of systems, such as regulation of the contraction of smooth muscle, the tuning of hair cells in the cochlea, regulation of transmitter release, and innate immunity. In smooth muscles, its activation by high level of Ca(2+), caused by ryanodine receptors in the sarcoplasmic reticulum, regulates the membrane potential. In cochlea cells, its number and kinetic properties partly determine the characteristic frequency of each hair cell and thereby helps to establish a tonotopic map. Kinetics of KCNMA1 channels are determined by alternative splicing, phosphorylation status and its combination with modulating beta subunits. Highly sensitive to both iberiotoxin (IbTx) and charybdotoxin (CTX).
- Gene Name:
- KCNMA1
- Uniprot ID:
- Q12791
- Molecular Weight:
- 137558.115 Da
References
- Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LM, Sanchez M, Giangiacomo K, Reuben JP, Smith AB 3rd, et al.: Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry. 1994 May 17;33(19):5819-28. [7514038 ]
- General Function:
- Potassium channel regulator activity
- Specific Function:
- Regulatory subunit of the calcium activated potassium KCNMA1 (maxiK) channel. Modulates the calcium sensitivity and gating kinetics of KCNMA1, thereby contributing to KCNMA1 channel diversity. Increases the apparent Ca(2+)/voltage sensitivity of the KCNMA1 channel. It also modifies KCNMA1 channel kinetics and alters its pharmacological properties. It slows down the activation and the deactivation kinetics of the channel. Acts as a negative regulator of smooth muscle contraction by enhancing the calcium sensitivity to KCNMA1. Its presence is also a requirement for internal binding of the KCNMA1 channel opener dehydrosoyasaponin I (DHS-1) triterpene glycoside and for external binding of the agonist hormone 17-beta-estradiol (E2). Increases the binding activity of charybdotoxin (CTX) toxin to KCNMA1 peptide blocker by increasing the CTX association rate and decreasing the dissociation rate.
- Gene Name:
- KCNMB1
- Uniprot ID:
- Q16558
- Molecular Weight:
- 21797.27 Da
References
- Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LM, Sanchez M, Giangiacomo K, Reuben JP, Smith AB 3rd, et al.: Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry. 1994 May 17;33(19):5819-28. [7514038 ]
- General Function:
- Potassium channel regulator activity
- Specific Function:
- Regulatory subunit of the calcium activated potassium KCNMA1 (maxiK) channel. Modulates the calcium sensitivity and gating kinetics of KCNMA1, thereby contributing to KCNMA1 channel diversity. Acts as a negative regulator that confers rapid and complete inactivation of KCNMA1 channel complex. May participate in KCNMA1 inactivation in chromaffin cells of the adrenal gland or in hippocampal CA1 neurons.
- Gene Name:
- KCNMB2
- Uniprot ID:
- Q9Y691
- Molecular Weight:
- 27129.37 Da
References
- Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LM, Sanchez M, Giangiacomo K, Reuben JP, Smith AB 3rd, et al.: Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry. 1994 May 17;33(19):5819-28. [7514038 ]
- General Function:
- Potassium channel regulator activity
- Specific Function:
- Regulatory subunit of the calcium activated potassium KCNMA1 (maxiK) channel. Modulates the calcium sensitivity and gating kinetics of KCNMA1, thereby contributing to KCNMA1 channel diversity. Alters the functional properties of the current expressed by the KCNMA1 channel. Isoform 2, isoform 3 and isoform 4 partially inactivate the current of KCNBMA. Isoform 4 induces a fast and incomplete inactivation of KCNMA1 channel that is detectable only at large depolarizations. In contrast, isoform 1 does not induce detectable inactivation of KCNMA1. Two or more subunits of KCNMB3 are required to block the KCNMA1 tetramer.
- Gene Name:
- KCNMB3
- Uniprot ID:
- Q9NPA1
- Molecular Weight:
- 31603.26 Da
References
- Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LM, Sanchez M, Giangiacomo K, Reuben JP, Smith AB 3rd, et al.: Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry. 1994 May 17;33(19):5819-28. [7514038 ]
- General Function:
- Potassium channel regulator activity
- Specific Function:
- Regulatory subunit of the calcium activated potassium KCNMA1 (maxiK) channel. Modulates the calcium sensitivity and gating kinetics of KCNMA1, thereby contributing to KCNMA1 channel diversity. Decreases the gating kinetics and calcium sensitivity of the KCNMA1 channel, but with fast deactivation kinetics. May decrease KCNMA1 channel openings at low calcium concentrations but increases channel openings at high calcium concentrations. Makes KCNMA1 channel resistant to 100 nM charybdotoxin (CTX) toxin concentrations.
- Gene Name:
- KCNMB4
- Uniprot ID:
- Q86W47
- Molecular Weight:
- 23948.465 Da
References
- Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LM, Sanchez M, Giangiacomo K, Reuben JP, Smith AB 3rd, et al.: Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry. 1994 May 17;33(19):5819-28. [7514038 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Gene Name:
- GABRA1
- Uniprot ID:
- P14867
- Molecular Weight:
- 51801.395 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA2
- Uniprot ID:
- P47869
- Molecular Weight:
- 51325.85 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA3
- Uniprot ID:
- P34903
- Molecular Weight:
- 55164.055 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA4
- Uniprot ID:
- P48169
- Molecular Weight:
- 61622.645 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- Transporter activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA5
- Uniprot ID:
- P31644
- Molecular Weight:
- 52145.645 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA6
- Uniprot ID:
- Q16445
- Molecular Weight:
- 51023.69 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- Ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Gene Name:
- GABRB1
- Uniprot ID:
- P18505
- Molecular Weight:
- 54234.085 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRB2
- Uniprot ID:
- P47870
- Molecular Weight:
- 59149.895 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- Gaba-gated chloride ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRB3
- Uniprot ID:
- P28472
- Molecular Weight:
- 54115.04 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRD
- Uniprot ID:
- O14764
- Molecular Weight:
- 50707.835 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRE
- Uniprot ID:
- P78334
- Molecular Weight:
- 57971.175 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRG1
- Uniprot ID:
- Q8N1C3
- Molecular Weight:
- 53594.49 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRG2
- Uniprot ID:
- P18507
- Molecular Weight:
- 54161.78 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRG3
- Uniprot ID:
- Q99928
- Molecular Weight:
- 54288.16 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. In the uterus, the function of the receptor appears to be related to tissue contractility. The binding of this pI subunit with other GABA(A) receptor subunits alters the sensitivity of recombinant receptors to modulatory agents such as pregnanolone.
- Gene Name:
- GABRP
- Uniprot ID:
- O00591
- Molecular Weight:
- 50639.735 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. Rho-1 GABA receptor could play a role in retinal neurotransmission.
- Gene Name:
- GABRR1
- Uniprot ID:
- P24046
- Molecular Weight:
- 55882.91 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. Rho-2 GABA receptor could play a role in retinal neurotransmission.
- Gene Name:
- GABRR2
- Uniprot ID:
- P28476
- Molecular Weight:
- 54150.41 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRR3
- Uniprot ID:
- A8MPY1
- Molecular Weight:
- 54271.1 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- Transmembrane signaling receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRQ
- Uniprot ID:
- Q9UN88
- Molecular Weight:
- 72020.875 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- G-protein coupled gaba receptor activity
- Specific Function:
- Component of a heterodimeric G-protein coupled receptor for GABA, formed by GABBR1 and GABBR2. Within the heterodimeric GABA receptor, only GABBR1 seems to bind agonists, while GABBR2 mediates coupling to G proteins. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors, such as adenylate cyclase. Signaling inhibits adenylate cyclase, stimulates phospholipase A2, activates potassium channels, inactivates voltage-dependent calcium-channels and modulates inositol phospholipid hydrolysis. Calcium is required for high affinity binding to GABA. Plays a critical role in the fine-tuning of inhibitory synaptic transmission. Pre-synaptic GABA receptor inhibits neurotransmitter release by down-regulating high-voltage activated calcium channels, whereas postsynaptic GABA receptor decreases neuronal excitability by activating a prominent inwardly rectifying potassium (Kir) conductance that underlies the late inhibitory postsynaptic potentials. Not only implicated in synaptic inhibition but also in hippocampal long-term potentiation, slow wave sleep, muscle relaxation and antinociception. Activated by (-)-baclofen, cgp27492 and blocked by phaclofen.Isoform 1E may regulate the formation of functional GABBR1/GABBR2 heterodimers by competing for GABBR2 binding. This could explain the observation that certain small molecule ligands exhibit differential affinity for central versus peripheral sites.
- Gene Name:
- GABBR1
- Uniprot ID:
- Q9UBS5
- Molecular Weight:
- 108319.4 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]
- General Function:
- G-protein coupled gaba receptor activity
- Specific Function:
- Component of a heterodimeric G-protein coupled receptor for GABA, formed by GABBR1 and GABBR2. Within the heterodimeric GABA receptor, only GABBR1 seems to bind agonists, while GABBR2 mediates coupling to G proteins. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors, such as adenylate cyclase. Signaling inhibits adenylate cyclase, stimulates phospholipase A2, activates potassium channels, inactivates voltage-dependent calcium-channels and modulates inositol phospholipid hydrolysis. Plays a critical role in the fine-tuning of inhibitory synaptic transmission. Pre-synaptic GABA receptor inhibits neurotransmitter release by down-regulating high-voltage activated calcium channels, whereas postsynaptic GABA receptor decreases neuronal excitability by activating a prominent inwardly rectifying potassium (Kir) conductance that underlies the late inhibitory postsynaptic potentials. Not only implicated in synaptic inhibition but also in hippocampal long-term potentiation, slow wave sleep, muscle relaxation and antinociception.
- Gene Name:
- GABBR2
- Uniprot ID:
- O75899
- Molecular Weight:
- 105820.52 Da
References
- Selala MI, Daelemans F, Schepens PJ: Fungal tremorgens: the mechanism of action of single nitrogen containing toxins--a hypothesis. Drug Chem Toxicol. 1989 Sep-Dec;12(3-4):237-57. [2698801 ]