| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2013-04-25 07:56:49 UTC |
|---|
| Update Date | 2014-12-24 20:26:32 UTC |
|---|
| Accession Number | T3D3789 |
|---|
| Identification |
|---|
| Common Name | Acibenzolar-S-Methyl |
|---|
| Class | Small Molecule |
|---|
| Description | Acibenzolar-S-methyl is a fungicide that works by activating a plant's own defense system by increasing the transcription of W-box controlled genes such as CAD1, NPR1 and PR2. It is a methyl derivative of acibenzolar. |
|---|
| Compound Type | - Ester
- Ether
- Fungicide
- Organic Compound
- Pesticide
- Synthetic Compound
|
|---|
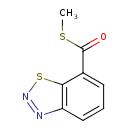
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1,2,3-Benzothiadiazole-7-carboxlic acid thiomethyl ester | | 7-(Methylthiocarbonyl)-benzo-1,2,3-thiadiazole | | Acibenzolar-S-methyl | | Actigard | | Benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester | | BTH | | S-Methyl benzo[1,2,3]thiadiazole-7-carbothioate |
|
|---|
| Chemical Formula | C8H6N2OS2 |
|---|
| Average Molecular Mass | 210.276 g/mol |
|---|
| Monoisotopic Mass | 209.992 g/mol |
|---|
| CAS Registry Number | 135158-54-2 |
|---|
| IUPAC Name | 1,2,3-benzothiadiazol-7-yl(methylsulfanyl)methanone |
|---|
| Traditional Name | acibenzolar-S-methyl |
|---|
| SMILES | CSC(=O)C1=CC=CC2=C1SN=N2 |
|---|
| InChI Identifier | InChI=1S/C8H6N2OS2/c1-12-8(11)5-3-2-4-6-7(5)13-10-9-6/h2-4H,1H3 |
|---|
| InChI Key | InChIKey=UELITFHSCLAHKR-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzothiadiazoles. These are heterocyclic aromatic compounds containing a benzene ring fused to a thiadiazole ring. Thiadiazole is a five-membered aromatic heterocycle made up of one sulfur atom and two nitrogen atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzothiadiazoles |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Benzothiadiazoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,2,3-benzothiadiazole
- Thiobenzoic acid or derivatives
- Benzenoid
- Azole
- Thiadiazole
- Heteroaromatic compound
- Carbothioic s-ester
- Thiocarboxylic acid ester
- Azacycle
- Sulfenyl compound
- Thiocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organopnictogen compound
- Organic oxygen compound
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 133°C | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0090000000-93a2e8d379c24617f1d6 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0290000000-625b556b6ede899460e2 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0012-6910000000-33b25e83272e21d55d11 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0390000000-af11f505a16a6872296d | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-1490000000-6535ae623ebd0f438a9d | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-06rx-7910000000-fdd7c9d5e7f6794d7852 | 2016-08-03 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, CDCl3, experimental) | Not Available | 2014-10-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100.40 MHz, CDCl3, experimental) | Not Available | 2014-10-20 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 86412 |
|---|
| ChEMBL ID | CHEMBL425055 |
|---|
| ChemSpider ID | 77928 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 73178 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D3789.pdf |
|---|
| General References | Not Available |
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|