| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2013-04-25 07:56:52 UTC |
|---|
| Update Date | 2014-12-24 20:26:33 UTC |
|---|
| Accession Number | T3D3871 |
|---|
| Identification |
|---|
| Common Name | Maleic hydrazide |
|---|
| Class | Small Molecule |
|---|
| Description | Maleic hydrazide (MH) was introduced into agriculture in the 1950s as a major commercial herbicide and a depressant of plant growth. It is a plant growth regulator (sprout inhibitor) and herbicide, that acts by inhibiting cell division in plants. It is used to control sprouting of potatoes and onions, suckers in tobacco, and growth of weeds, grasses and trees in/along lawns, turf, ornamental plants, non-bearing citrus, utility and highway rights-of-way, airports and industrial land. Most of the maleic hydrazide used in the U.S. is applied to tobacco (86-88%), followed by potatoes (10%), It is used to control sucker growth on tobacco plants, retardation of flowering and prolongation of dormancy period. |
|---|
| Compound Type | - Ester
- Herbicide
- Industrial/Workplace Toxin
- Organic Compound
- Pesticide
- Synthetic Compound
|
|---|
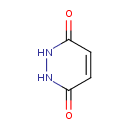
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1,2,3,6-Tetrahydro-3,6-dioxopyridazine | | 1,2-Dihydro-3,6-pyridazinedione | | 1,2-dihydropyridazine-3,6-dione | | 3,6-Dihydroxypyridazine | | 3,6-Pyridazinediol | | Antergon | | Antyrost | | Burtolin | | cis-Butenedioic acid hydrazide | | De-cut | | De-sprout | | Drexel-super P | | Fair plus | | Fazor | | Gotax | | Maintain 3 | | Malein 30 | | Malepin | | Malzid | | Mazide | | Milurit | | N,N-Maleoylhydrazine | | Regulox | | Retard | | Sorbatran | | Sprout-stop | | Sucker-stuff | | Super-de-sprout | | Unriprim | | Vondrax |
|
|---|
| Chemical Formula | C4H4N2O2 |
|---|
| Average Molecular Mass | 112.087 g/mol |
|---|
| Monoisotopic Mass | 112.027 g/mol |
|---|
| CAS Registry Number | 123-33-1 |
|---|
| IUPAC Name | 1,2,3,6-tetrahydropyridazine-3,6-dione |
|---|
| Traditional Name | stuntman |
|---|
| SMILES | O=C1NNC(=O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C4H4N2O2/c7-3-1-2-4(8)6-5-3/h1-2H,(H,5,7)(H,6,8) |
|---|
| InChI Key | InChIKey=BGRDGMRNKXEXQD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyridazinones. Pyridazinones are compounds containing a pyridazine ring which bears a ketone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyridazines and derivatives |
|---|
| Direct Parent | Pyridazinones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyridazinone
- Heteroaromatic compound
- Lactam
- Azacycle
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03di-9500000000-f5330323db43f9bb6ad2 | 2021-09-23 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0900000000-404b6cd62e19c5a04c10 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-4900000000-38a7721dc374a821eb53 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01tc-9100000000-81dcd3af33e231125c4a | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01ox-9400000000-34632b70bc6d26797f6f | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9200000000-319866a718598df7f88d | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000x-9000000000-4b04b8bb9129d8150deb | 2016-08-03 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-03e9-9400000000-5660402a90273ab567b0 | 2014-10-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 90 MHz, DMSO-d6, experimental) | Not Available | 2014-10-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 15.09 MHz, DMSO-d6, experimental) | Not Available | 2014-10-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-10-27 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 3, not classifiable as to its carcinogenicity to humans. (1) |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 21954 |
|---|
| ChEMBL ID | CHEMBL1489913 |
|---|
| ChemSpider ID | 20632 |
|---|
| KEGG ID | C18474 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D3871.pdf |
|---|
| General References | - International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|