| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-08-29 05:04:05 UTC |
|---|
| Update Date | 2014-12-24 20:26:38 UTC |
|---|
| Accession Number | T3D4081 |
|---|
| Identification |
|---|
| Common Name | Matrine |
|---|
| Class | Small Molecule |
|---|
| Description | Matrine is an alkaloid found in plants from the Sophora genus. It has a variety of pharmacological effects, including anti-cancer effects, and action as a kappa opioid receptor and μ-receptor agonist. |
|---|
| Compound Type | - Amide
- Amine
- Natural Compound
- Organic Compound
- Plant Toxin
|
|---|
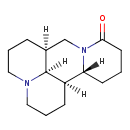
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (+)-Matrine | | Matridin-15-one | | Sophoridine |
|
|---|
| Chemical Formula | C15H24N2O |
|---|
| Average Molecular Mass | 248.364 g/mol |
|---|
| Monoisotopic Mass | 248.189 g/mol |
|---|
| CAS Registry Number | 519-02-8 |
|---|
| IUPAC Name | (1R,2R,9S,17S)-7,13-diazatetracyclo[7.7.1.0²,⁷.0¹³,¹⁷]heptadecan-6-one |
|---|
| Traditional Name | matrine |
|---|
| SMILES | [H][C@]12CCCN3CCC[C@]([H])([C@@]4([H])CCCC(=O)N4C1)[C@]23[H] |
|---|
| InChI Identifier | InChI=1S/C15H24N2O/c18-14-7-1-6-13-12-5-3-9-16-8-2-4-11(15(12)16)10-17(13)14/h11-13,15H,1-10H2/t11-,12+,13+,15-/m0/s1 |
|---|
| InChI Key | InChIKey=ZSBXGIUJOOQZMP-JLNYLFASSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as matrine alkaloids. These are lupin alkaloids with a structure based on the matrine skeleton, a four-ring skeleton that based on a saturated dipyrido[2,1-f:3',2',1'-ij][1,6]naphthyridin-10-one skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Lupin alkaloids |
|---|
| Sub Class | Matrine alkaloids |
|---|
| Direct Parent | Matrine alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Matrine
- Quinolizidinone
- Diazanaphthalene
- Naphthyridine
- Quinolizidine
- Delta-lactam
- Piperidinone
- Piperidine
- Tertiary carboxylic acid amide
- Tertiary aliphatic amine
- Tertiary amine
- Amino acid or derivatives
- Carboxamide group
- Lactam
- Organoheterocyclic compound
- Azacycle
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organic oxygen compound
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Amine
- Carbonyl group
- Organopnictogen compound
- Organic oxide
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cell surface
- Cytoplasm
- Cytosol
- Endoplasmic reticulum
- Extracellular
- Extracellular matrix
- Lysosome
- Mitochondrion
- Plasma Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Apoptosis | Not Available | map04210 | | Cell cycle | Not Available | map04110 | | Gastric acid secretion | Not Available | map04971 | | Nf-kappa b signaling pathway | Not Available | map04064 |
|
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-8ee63c2a1c35de3fff22 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0090000000-4f7ecba727c2ca8097ca | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fbc-2930000000-b8e4ecbc12721437841b | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-274cc1f6bff7ae2cfb7c | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-dc7aab170fe29e23e17a | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0036-5970000000-88a546869c5bb0c632d9 | 2016-08-03 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-052b-9630000000-eed96cedc2334a0b0d1e | 2014-09-20 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | The nervous system is the main target organ by the toxicity of matrine. Morphological observation revealed degenerative changes of the nerve cells in the brain tissue of the mice. (1) Matrine has a variety of pharmacological effects, including anti-cancer effects, and action as a kappa opioid receptor and µ-receptor agonist. Matrine possesses strong antitumor activities in vitro and in vivo. Inhibition of cell proliferation and induction of apoptosis are the likely mechanisms responsible for matrine's antitumor activities. (Wikipedia) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Matrine is an alkaloid found in plants from the Sophora genus. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 91466 |
|---|
| ChEMBL ID | CHEMBL204860 |
|---|
| ChemSpider ID | 82591 |
|---|
| KEGG ID | C10774 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D4081.pdf |
|---|
| General References | - Wang XY, Liang L, Chang JL, Yang MH, Li ZG: [Toxicity of matrine in Kunming mice]. Nan Fang Yi Ke Da Xue Xue Bao. 2010 Sep;30(9):2154-5. [20855277 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|