| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-08-29 05:52:38 UTC |
|---|
| Update Date | 2020-07-08 16:41:42 UTC |
|---|
| Accession Number | T3D4203 |
|---|
| Identification |
|---|
| Common Name | Furan |
|---|
| Class | Small Molecule |
|---|
| Description | Furan is a member of the class of compounds known as furans. These are molecules containing a heterocyclic organic group consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. Furan is aromatic because one of the lone pairs of electrons on the oxygen atom is delocalized into the ring, creating a 4n+2 aromatic system similar to benzene. Because of the aromaticity, furan is flat and lacks discrete double bonds. Furan is a colourless, flammable, highly volatile liquid with a boiling point close to room temperature (31°C). It is soluble in common organic solvents, including alcohol, ether, and acetone, but is insoluble in water. It has a strong ethereal odour. Furan is found in heat-treated (e.g. cooked, roasted, baked, pasteurized, and sterilized) commercial foods and is produced through thermal degradation of natural food constituents (PMID: 22641279). It can be found in roasted coffee, instant coffee, and processed baby foods (PMID: 22641279). In particular, the highest furan levels can be detected in coffee, with mean values between 42 and 3 660 ng/g for brewed coffee and roasted coffee beans. Furan can also be detected at levels between 0.2 and 3.2 ng/g in infant formula, from 22 to 24 ng/g in baked beans, from 13 to 17 ng/g in meat products, and from 23 to 24 ng/g in soups. In soy sauce, furan is detectable at 27 ng/g (PMID: 26483883). Research has indicated that coffee made in espresso makers and, above all, coffee made from capsules, contains more furan than that made in traditional drip coffee makers, although the levels are still within safe health limits. Various pathways have been reported for the formation of furan: (1) thermal degradation and/or thermal rearrangement of carbohydrates in the presence of amino acids, (2) thermal degradation of certain amino acids (aspartic acid, threonine, α-alanine, serine, and cysteine), (3) oxidation of ascorbic acid at higher temperatures, and (4) oxidation of polyunsaturated fatty acids and carotenoids (PMID: 26483883). Several studies have reported that furan formation occurs to a large extent during the Maillard reaction. The Maillard reaction involves the thermal degradation and rearrangement of carbohydrates (i.e. non-enzymatic browning reactions during food processing and cooking). Reducing hexoses often go through the Maillard reaction in the presence of amino acids and produce reactive intermediates such as 1-deoxy- and 3-deoxyosones, aldotetrose, and 2-deoxy-3-keto-aldotetrose. 2-Deoxy-3-keto-aldotetrose typically goes through retro-aldol cleavage leading to 3-deoxyosone which undergoes α-dicarbonyl cleavage, followed by oxidation and decarboxylation to form 2-deoxyaldotetrose, which is a direct precursor of furan. In addition to the formation of furan via carbohydrate degradation, furan can also be formed through thermal degradation of certain amino acids. Specifically, the amino acids that can form acetaldehyde and glycolaldehyde can produce furan by aldol condensation and cyclization (PMID: 26483883). Furan is toxic and may be carcinogenic. In particular, furan is a potent hepatotoxin and hepatocarcinogen in rodents, causing hepatocellular adenomas and carcinomas in rats and mice, and high incidences of cholangiocarcinomas in rats at doses ≥ 2 mg/kg (PMID: 22641279). |
|---|
| Compound Type | - Cigarette Toxin
- Industrial/Workplace Toxin
- Metabolite
- Organic Compound
- Solvent
- Synthetic Compound
|
|---|
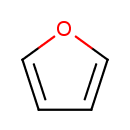
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1,4-Epoxy-1,3-butadiene | | Divinylene oxide | | Furane | | Furfuran | | Oxacyclopentadiene | | Oxole | | Tetrole |
|
|---|
| Chemical Formula | C4H4O |
|---|
| Average Molecular Mass | 68.074 g/mol |
|---|
| Monoisotopic Mass | 68.026 g/mol |
|---|
| CAS Registry Number | 110-00-9 |
|---|
| IUPAC Name | furan |
|---|
| Traditional Name | furan |
|---|
| SMILES | O1C=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C4H4O/c1-2-4-5-3-1/h1-4H |
|---|
| InChI Key | InChIKey=YLQBMQCUIZJEEH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as heteroaromatic compounds. Heteroaromatic compounds are compounds containing an aromatic ring where a carbon atom is linked to an hetero atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Heteroaromatic compounds |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Heteroaromatic compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Heteroaromatic compound
- Furan
- Oxacycle
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | |
|---|
| Chemical Roles | |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | -85.6°C | | Boiling Point | 32°C (89.6°F) | | Solubility | 10 mg/mL at 25°C | | LogP | 1.34 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-00kr-9000000000-4c50652f33a401ab3cae | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-00kr-9000000000-d007e0256578ac930b22 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-00kr-9000000000-4c50652f33a401ab3cae | 2018-05-18 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-00kr-9000000000-d007e0256578ac930b22 | 2018-05-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-9000000000-511af11e910184693aa1 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-9000000000-fd05695071ee559e9be4 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9000000000-208e5dd03db999e390ff | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-9000000000-351aa9d338efe69d31ef | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-9000000000-acebecda0e70274875b1 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-9000000000-1df91dbb0164452d0fd1 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014u-9000000000-41895048238024d76bc7 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ku-9000000000-a223589b20bc67c79fe0 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udr-9000000000-056962459138c52c54c8 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00kf-9000000000-983ab9ff6334b0b9a584 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00ke-9000000000-77469f3e4a080d7d3681 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kg-9000000000-d4fe1a89a559171ed26a | 2021-09-22 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-00kr-9000000000-3bddc12b5a8dd3ca188d | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 90 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 25.16 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Furan is a potent hepatotoxin and hepatocarcinogen in rodents, causing hepatocellular adenomas and carcinomas in rats and mice, and high incidences of cholangiocarcinomas in rats at doses ≥ 2 mg/kg bw. A genotoxic mode of action cannot be excluded for furan-induced tumor formation. Furan is metabolized by cytochrome P450 (CYP) enzymes, predominantly CYP2E1, to its major metabolite cis-2-butene-1,4-dial (BDA, maleic dialdehyde), a highly reactive electrophile identified as the key mediator of furan toxicity and carcinogenicity. Furan-mediated effects on glutathione (GSH) levels and cell viability can be suppressed by the CYP inhibitor 1-phenylimidazole and increased by pretreatment of rats with acetone (a CYP2E1-inducing agent), indicating that furan cytotoxicity depends on its metabolic activation. BDA has been shown to react with cellular nucleophiles such as GSH and amino acids and to cause cross-links between thiols and amino groups, giving rise to lactam and pyrrole derivatives. Furan reduced the percentage of DNA in the comet tail in turkey liver fetal hepatocytes. Furan was also shown to induce chromosomal aberrations and sister chromatid exchanges (SCEs) in Chinese hamster ovary (CHO) cells. A statistically significant increase of micronucleated cells was recently reported in the spleen of furan-treated mice. A reduction of percentage of DNA in comet tail in liver cells was observed following treatment of turkey fetuses in ovo. Exposure to furan at doses associated with increased tumor incidences initially causes hepatocellular necrosis, accompanied by inflammation and sustained regenerative proliferation of hepatocytes, which may present key events in furan-induced hepatocellular carcinogenicity. Subcapsular and centrilobular necrosis accompanied by markedly increased liver enzymes is the primary response to furan treatment. The involvement of inflammatory processes in furan toxicity is also reflected by increased expression of cytokines and other inflammation-associated genes, such as IFN-γ, IL-1β, IL-6, IL-10, and components of the complement system, which may, however, also derive from lesions involving the biliary tract. Indeed, increased production of reactive oxygen species in response to furan is suggested by immunohistochemical detection of 8-oxo-dG within nuclei of hepatocytes of centrilobular areas following high-dose exposure and changes in the expression of genes responsive to oxidative stress in rats and/or mice. (1) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 2B, possibly carcinogenic to humans. (2) |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB13785 |
|---|
| PubChem Compound ID | 8029 |
|---|
| ChEMBL ID | CHEMBL278980 |
|---|
| ChemSpider ID | 7738 |
|---|
| KEGG ID | C14275 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 35559 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | SUC |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Furan |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Link |
|---|
| General References | - Moro S, Chipman JK, Wegener JW, Hamberger C, Dekant W, Mally A: Furan in heat-treated foods: formation, exposure, toxicity, and aspects of risk assessment. Mol Nutr Food Res. 2012 Aug;56(8):1197-211. doi: 10.1002/mnfr.201200093. Epub 2012 May 29. [22641279 ]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|