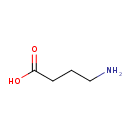
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-00dj-1900000000-f831f79dfcaeffa8b177 | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-00di-1900000000-2de9d92a2cfc7bc655f4 | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-00di-1900000000-73bbf2ee0803f058dbed | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-00di-1901000000-85d4bd98af8534428b5a | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-00di-0900000000-6be23968e972a414be51 | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-00di-1900000000-9a224763afd8ca892add | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-00di-9800000000-d8906d09ca1872a6391c | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-0udi-1900000000-54db7e21790401045519 | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (3 TMS) | splash10-00di-1901000000-b047af158215c2b5b8e8 | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-001i-9000000000-21ea76dfb0da62031f1d | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-00di-0901000000-5d60b0a446fd8122f613 | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00dj-1900000000-f831f79dfcaeffa8b177 | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00di-1900000000-2de9d92a2cfc7bc655f4 | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00di-1900000000-73bbf2ee0803f058dbed | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00di-1901000000-85d4bd98af8534428b5a | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00di-0900000000-6be23968e972a414be51 | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00di-1900000000-9a224763afd8ca892add | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00di-9800000000-d8906d09ca1872a6391c | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0udi-1900000000-54db7e21790401045519 | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-00di-1901000000-b047af158215c2b5b8e8 | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00dj-1900000000-1219470a0be188da64e6 | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0udi-0900000000-f7117dfaf9d856c95919 | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0006-1900000000-e35585a985d8128d044e | 2017-09-12 | View Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-9000000000-d5f55a414ff1e8c65d9d | 2016-09-22 | View Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0fk9-9700000000-4b2809309587240dd479 | 2017-10-06 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0uxr-8900000000-ce0d8f44422836cd9965 | 2012-07-24 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0005-9000000000-8ce5afb97d7c08821da3 | 2012-07-24 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0005-9100000000-32c433b2c916697690f8 | 2012-07-24 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0udi-0900000000-5831aaabdf53f3132ae5 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0a4i-9000000000-9babfd4a6937ecba7318 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0a4i-9000000000-e1c0c1485d846e9b123b | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0udi-0900000000-647d55ecf98850e7a875 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0udi-0900000000-7c107641a38922c88fca | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-000i-9000000000-86718b349efad6334e3a | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-000i-9000000000-a1e84e55e4b6c6628d5d | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0006-0009000000-29c22bf0ed844d09c0af | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-0udi-0900000000-1d00adad47e42c60c340 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-0udi-1900000000-47b195fb74720cc99464 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-001i-9000000000-a14a52dc59bf9988bb44 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive | splash10-0udi-5900000000-20c55b2809389d5ad83b | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive | splash10-000i-9000000000-eca4c5aefca98751a11e | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive | splash10-014j-9000000000-f0783316e09291749409 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive | splash10-0005-9000000000-81837eb9c0b926cb0e81 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive | splash10-0005-9000000000-8b48126992d7fa242636 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-000i-9000000000-7d4636efbc4e5d75872e | 2012-08-31 | View Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-9100000000-655f9d93a35bfa537583 | 2015-05-27 | View Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ku-9000000000-4a334d5e272576f62403 | 2015-05-27 | View Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-4a13b03446b3370ccd43 | 2015-05-27 | View Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-9100000000-655f9d93a35bfa537583 | 2015-05-27 | View Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ku-9000000000-4a334d5e272576f62403 | 2015-05-27 | View Spectrum |
| MS | Mass Spectrum (Electron Ionization) | splash10-001i-9000000000-dbf4f9e19a35f953a189 | 2014-09-20 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | 2012-12-04 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 125 MHz, H2O, experimental) | Not Available | 2012-12-04 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 90 MHz, D2O, experimental) | Not Available | 2014-09-20 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 25.16 MHz, D2O, experimental) | Not Available | 2014-09-23 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, D2O, experimental) | Not Available | 2016-10-22 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | 2012-12-04 | View Spectrum |