| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-08-29 06:25:21 UTC |
|---|
| Update Date | 2018-03-21 17:46:22 UTC |
|---|
| Accession Number | T3D4322 |
|---|
| Identification |
|---|
| Common Name | Uroporphyrin III |
|---|
| Class | Small Molecule |
|---|
| Description | Uroporphyrin is the porphyrin produced by oxidation of the methylene bridges in uroporphyrinogen. Uroporphyrins have four acetic acid and four propionic acid side chains attached to their pyrrole rings. The enzyme uroporphyrinogen I synthase catalyzes the formation of hydroxymethylbilane from four molecules of porphobilinogen. Uroporphyrinogen III cosynthase then catalyzes the conversion of hydroxymethylbilane into uroporphyrinogen III. Otherwise, hydroxymethylbilane cyclizes nonenzymatically to form uroporphyrinogen I. Uroporphyrinogen I and III yield their respective uroporphyrins via autooxidation or their respective coproporphyrinogens via decarboxylation. Excessive amounts of uroporphyrin I are excreted in congenital erythropoietic porphyria, and both uroporphyrin I and uroporphyrin III are excreted in porphyria cutanea tarda. Uroporphyrin I and III are the most common isomers. Under certain conditions, uroporphyrin III can act as a phototoxin, a neurotoxin, and a metabotoxin. A phototoxin leads to cell damage upon exposure to light. A neurotoxin causes damage to nerve cells and nerve tissues. A metabotoxin is an endogenously produced metabolite that causes adverse health effects at chronically high levels. Chronically high levels of porphyrins are associated with porphyrias such as porphyria variegate, acute intermittent porphyria, porphyria cutanea tarda, and hereditary coproporphyria (HCP). There are several types of porphyrias (most are inherited). Hepatic porphyrias are characterized by acute neurological attacks (seizures, psychosis, extreme back and abdominal pain, and an acute polyneuropathy), while the erythropoietic forms present with skin problems (usually a light-sensitive blistering rash and increased hair growth). The neurotoxicity of porphyrins may be due to their selective interactions with tubulin, which disrupt microtubule formation and cause neural malformations (PMID: 3441503). |
|---|
| Compound Type | - Animal Toxin
- Food Toxin
- Metabolite
- Natural Compound
- Organic Compound
|
|---|
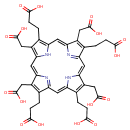
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 3,8,13,17-Tetrakis(carboxymethyl)porphyrin-2,7,12,18-tetrapropanoate | | 3,8,13,17-Tetrakis(carboxymethyl)porphyrin-2,7,12,18-tetrapropanoic acid | | 3,8,13,17-Tetramethyl-2,7,12,18-Porphinetetrapropionate | | 3,8,13,17-Tetramethyl-2,7,12,18-Porphinetetrapropionic acid | | Coproporphyrin III |
|
|---|
| Chemical Formula | C40H38N4O16 |
|---|
| Average Molecular Mass | 830.747 g/mol |
|---|
| Monoisotopic Mass | 830.228 g/mol |
|---|
| CAS Registry Number | 18273-06-8 |
|---|
| IUPAC Name | 3-[9,14,20-tris(2-carboxyethyl)-5,10,15,19-tetrakis(carboxymethyl)-21,22,23,24-tetraazapentacyclo[16.2.1.1^{3,6}.1^{8,11}.1^{13,16}]tetracosa-1,3,5,7,9,11(23),12,14,16,18(21),19-undecaen-4-yl]propanoic acid |
|---|
| Traditional Name | 3-[9,14,20-tris(2-carboxyethyl)-5,10,15,19-tetrakis(carboxymethyl)-21,22,23,24-tetraazapentacyclo[16.2.1.1^{3,6}.1^{8,11}.1^{13,16}]tetracosa-1,3,5,7,9,11(23),12,14,16,18(21),19-undecaen-4-yl]propanoic acid |
|---|
| SMILES | [H]\C-1=C2\N\C(=C([H])/C3=N/C(=C([H])\C4=C(CCC(O)=O)C(CC(O)=O)=C(N4)\C([H])=C4/N=C-1C(CCC(O)=O)=C4CC(O)=O)/C(CC(O)=O)=C3CCC(O)=O)C(CC(O)=O)=C2CCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C40H38N4O16/c45-33(46)5-1-17-21(9-37(53)54)29-14-27-19(3-7-35(49)50)22(10-38(55)56)30(43-27)15-28-20(4-8-36(51)52)24(12-40(59)60)32(44-28)16-31-23(11-39(57)58)18(2-6-34(47)48)26(42-31)13-25(17)41-29/h13-16,41,44H,1-12H2,(H,45,46)(H,47,48)(H,49,50)(H,51,52)(H,53,54)(H,55,56)(H,57,58)(H,59,60)/b25-13-,26-13-,27-14-,28-15-,29-14-,30-15-,31-16-,32-16- |
|---|
| InChI Key | InChIKey=VZVFNUAIRVUCEW-UJJXFSCMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as porphyrins. Porphyrins are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Porphyrins |
|---|
| Direct Parent | Porphyrins |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Endogenous |
|---|
| Cellular Locations | - Cytoplasm
- Membrane
- Mitochondria
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | |
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Porphyrin Metabolism | SMP00024 | map00860 | | Congenital Erythropoietic Porphyria (CEP) or Gunther Disease | SMP00345 | Not Available | | Porphyria Variegata (PV) | SMP00346 | Not Available |
|
|---|
| Applications | Not Available |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0hft-0000000920-cc03b9bed8ec1e3756f2 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gbi-0000000900-b9c5416788c41a1b932d | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ftr-0000000900-ddf789a09baa29543062 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-02t9-0000000940-171f6daf64defe53ba6e | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0000000910-e893ced4f4069e732360 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-2000000900-e8e4a229f7baf3810285 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014r-0000000900-1ba54d2ecd921bbf981c | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014u-0000000900-8dfb85a7347e408fafae | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00ku-0000000900-cc696857ebd0630cc06a | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-02t9-0000000930-9dd5499b3b084b8cef39 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00or-0000000900-e6018a2ba33c9cad4454 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0173-0000000900-24c512158030a2f09176 | 2021-09-23 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | Not Available | 2022-08-06 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is an endogenously produced metabolite found in the human body. It is used in metabolic reactions, catabolic reactions or waste generation. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Chronically high levels of porophyrins are associated with porphyrias such as Porphyria variegate, Acute Intermittent Porphyria and Hereditary Coproporphyria (HCP). |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB04461 |
|---|
| HMDB ID | HMDB00916 |
|---|
| PubChem Compound ID | Not Available |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 16736727 |
|---|
| KEGG ID | C02469 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 15436 |
|---|
| BioCyc ID | UROPORPHYRIN_III |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | UFE |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Ichiro Kojima, Kenji Maruhashi, Yasuo Fujiwara, “Process for producing coproporphyrin III.” U.S. Patent US4334021, issued September, 1978. |
|---|
| MSDS | T3D4322.pdf |
|---|
| General References | - Tsai SF, Bishop DF, Desnick RJ: Purification and properties of uroporphyrinogen III synthase from human erythrocytes. J Biol Chem. 1987 Jan 25;262(3):1268-73. [3805019 ]
- Bozek P, Hutta M, Hrivnakova B: Rapid analysis of porphyrins at low ng/l and microg/l levels in human urine by a gradient liquid chromatography method using octadecylsilica monolithic columns. J Chromatogr A. 2005 Aug 19;1084(1-2):24-32. [16114232 ]
- Ohdoi C, Nyhan WL, Kuhara T: Chemical diagnosis of Lesch-Nyhan syndrome using gas chromatography-mass spectrometry detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 Jul 15;792(1):123-30. [12829005 ]
- Luo J, Lim CK: Isolation and characterization of new porphyrin metabolites in human porphyria cutanea tarda and in rats treated with hexachlorobenzene by HPTLC, HPLC and liquid secondary ion mass spectrometry. Biomed Chromatogr. 1995 May-Jun;9(3):113-22. [7655298 ]
- Schonning C, Leeming R, Stenstrom TA: Faecal contamination of source-separated human urine based on the content of faecal sterols. Water Res. 2002 Apr;36(8):1965-72. [12092571 ]
- Hernandez-Zavala A, Del Razo LM, Garcia-Vargas GG, Aguilar C, Borja VH, Albores A, Cebrian ME: Altered activity of heme biosynthesis pathway enzymes in individuals chronically exposed to arsenic in Mexico. Arch Toxicol. 1999 Mar;73(2):90-5. [10350189 ]
- Salen G, Berginer V, Shore V, Horak I, Horak E, Tint GS, Shefer S: Increased concentrations of cholestanol and apolipoprotein B in the cerebrospinal fluid of patients with cerebrotendinous xanthomatosis. Effect of chenodeoxycholic acid. N Engl J Med. 1987 May 14;316(20):1233-8. [3106810 ]
- To-Figueras J, Ozalla D, Mateu CH: Long-standing changes in the urinary profile of porphyrin isomers after clinical remission of porphyria cutanea tarda. Ann Clin Lab Sci. 2003 Summer;33(3):251-6. [12956438 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|