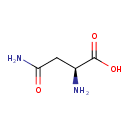
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-0159-0910000000-bedf57998656ab5ebc16 | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-0159-0910000000-4979c4d028d2dc931263 | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-0159-0910000000-b7ea3fef61f3940cbdda | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-00di-9710000000-6d0afcbcc003347e598e | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-0uxr-0910000000-2be567239bd3229b1ca1 | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-02t9-0790000000-b8141f48cbebb90f683e | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-02t9-1790000000-1d12ed9b4fb2799da766 | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (4 TMS) | splash10-014u-0961000000-65a5c0999f17110c9939 | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (4 TMS) | splash10-00kr-0940000000-0e0e5c7bdbac5ea0e49e | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (3 TMS) | splash10-00lr-1920000000-7864dbb1f685e64dd1cf | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (4 TMS) | splash10-000i-1940000000-964ea25da2789805985a | 2014-06-16 | View Spectrum |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-00lr-0930000000-79a089d9875e092dd7f2 | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0159-0910000000-bedf57998656ab5ebc16 | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0159-0910000000-4979c4d028d2dc931263 | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0159-0910000000-b7ea3fef61f3940cbdda | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-QQ (Non-derivatized) | splash10-00di-4931100000-17f149310ce3a0f748d1 | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00di-9710000000-6d0afcbcc003347e598e | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0uxr-0910000000-2be567239bd3229b1ca1 | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-02t9-0790000000-b8141f48cbebb90f683e | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-02t9-1790000000-1d12ed9b4fb2799da766 | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-014u-0961000000-65a5c0999f17110c9939 | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-00kr-0940000000-0e0e5c7bdbac5ea0e49e | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-00lr-1920000000-7864dbb1f685e64dd1cf | 2017-09-12 | View Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-000i-1940000000-964ea25da2789805985a | 2017-09-12 | View Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000f-9100000000-5881591331bd059b7e7d | 2016-09-22 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0080-9400000000-e5c7e19f427eea6d71b0 | 2012-07-24 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-00di-9000000000-afc1214100db1168b095 | 2012-07-24 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-00dl-9000000000-57e977cd87e9a86d482b | 2012-07-24 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-001i-0900000000-6fb96f5aa291359dba29 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-000i-9000000000-76f11e6fe5657c35d15f | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-000i-9000000000-e944486273dbfde4cae4 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-001i-0900000000-27ad91a86be0c4d86c3c | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-001i-0900000000-b8b7a3431b66246ad613 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-000i-9000000000-03fa294ec740e189dd99 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-000i-9000000000-cee2081406fc25ab6169 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-001i-0900000000-cd79afa0c27f65e54adc | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-01q9-0943200000-5a24322ba0ce2f410155 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-03di-0900000000-fc3086cc1bb0c06a4c11 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-03di-3900000000-2d74d8232e7523f3b1c9 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-001i-0900000000-fa61c6fa9a87ce4905bb | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-01q9-0942200000-757da8b3406485c997b0 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-014i-9000000000-eac18512f2d22ab7a7ef | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-03di-0900000000-8df6d5b6cf94bf081c89 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-001i-0900000000-18079004a95c252da208 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-001i-0900000000-e6b1f9b4982e6cdc863d | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-03di-5900000000-7b9c09b3b6de28972f97 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-00dl-9100000000-2ea95ead344d62e230a4 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-0006-9000000000-defacc365589bc4437d7 | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-0006-9000000000-fd60db4d5e35794c45ae | 2012-08-31 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive | splash10-001i-3900000000-fd0f1034b5c1e40435c0 | 2012-08-31 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | Not Available | 2012-12-04 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 125 MHz, H2O, experimental) | Not Available | 2012-12-04 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, D2O, experimental) | Not Available | 2016-10-22 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, experimental) | Not Available | 2021-10-10 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | Not Available | 2021-10-10 | View Spectrum |
| 2D NMR | [1H, 1H]-TOCSY. Unexported temporarily by An Chi on Oct 15, 2021 until json or nmrML file is generated. 2D NMR Spectrum (experimental) | Not Available | 2012-12-04 | View Spectrum |
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | 2012-12-05 | View Spectrum |