| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-08-29 06:51:38 UTC |
|---|
| Update Date | 2014-12-24 20:26:51 UTC |
|---|
| Accession Number | T3D4481 |
|---|
| Identification |
|---|
| Common Name | Butylate |
|---|
| Class | Small Molecule |
|---|
| Description | Butylate is one of the pesticide compounds considered by the US EPA to have the greatest potential for leaching into groundwater (9). Butylate is only slightly soluble in water (46 micrograms/ml). It degrades fairly rapidly with a soil half life of 3 to 10 weeks in moist soils under aerobic conditions. The major routes of exposure to butylate are through the skin and by inhalation. Butylate is a thiocarbamate, a class of chemicals known for their tendency to irritate the skin and the mucous membranes of the respiratory tract. It may cause symptoms of scratchy throat, sneezing, and coughing when large amounts of dusts or spray are inhaled (4,7). Slight eye irritation can be caused by butylate, potentially leading to permanent eye damage [7,22]. Skin irritation was observed in rabbits topically exposed to 2000 mg of technical butylate (85.71% pure) for 24 hours. |
|---|
| Compound Type | - Ether
- Lachrymator
- Organic Compound
- Pesticide
- Synthetic Compound
|
|---|
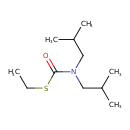
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Butylic acid | | S-Ethyl N,N-diisobutylthiocarbamate | | Sutan |
|
|---|
| Chemical Formula | C11H23NOS |
|---|
| Average Molecular Mass | 217.371 g/mol |
|---|
| Monoisotopic Mass | 217.150 g/mol |
|---|
| CAS Registry Number | 2008-41-5 |
|---|
| IUPAC Name | N,N-bis(2-methylpropyl)(ethylsulfanyl)formamide |
|---|
| Traditional Name | sutan |
|---|
| SMILES | CCSC(=O)N(CC(C)C)CC(C)C |
|---|
| InChI Identifier | InChI=1S/C11H23NOS/c1-6-14-11(13)12(7-9(2)3)8-10(4)5/h9-10H,6-8H2,1-5H3 |
|---|
| InChI Key | InChIKey=BMTAFVWTTFSTOG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thiocarbamic acid derivatives. These are organic compounds containing a functional group with the general structure OC(=S)NR2 or SC(=O)NR2. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organosulfur compounds |
|---|
| Class | Thiocarbonyl compounds |
|---|
| Sub Class | Thiocarbamic acid derivatives |
|---|
| Direct Parent | Thiocarbamic acid derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Thiocarbamic acid derivative
- Carbonic acid derivative
- Sulfenyl compound
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Membrane
- Microsome
- Nuclear Membrane
- Nucleolus
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Cell cycle | Not Available | map04110 |
|
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a6u-9400000000-d1d1f8a6c8bfa173c16a | 2021-09-24 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-066r-9480000000-2ac96ac9b16bf3838896 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9410000000-23fd72c1bbe4a577f140 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-76a51ccaa93b3863b2cb | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0gbi-4970000000-503dcc3ffb6894937bf1 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0h00-6910000000-2356c5cedd154cbf50a3 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9400000000-6015564e4a5bb9efc668 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-07vi-1940000000-c7d6318d68b460f4ee68 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0r0r-4910000000-eedd0a5a6e716cdf099e | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-9200000000-faa172c764f5a456d330 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0920000000-32b018094f595c34e860 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056r-4910000000-7f07f9ae7887740ca441 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9200000000-8ad0c6f929f8756649c1 | 2021-10-12 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0a4i-9400000000-172256c4a90240178455 | 2014-09-20 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 16181 |
|---|
| ChEMBL ID | CHEMBL1873329 |
|---|
| ChemSpider ID | 15357 |
|---|
| KEGG ID | C14323 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D4481.pdf |
|---|
| General References | - Extension Toxicology Network [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|