| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-08-29 06:51:42 UTC |
|---|
| Update Date | 2014-12-24 20:26:51 UTC |
|---|
| Accession Number | T3D4501 |
|---|
| Identification |
|---|
| Common Name | Ethametsulfuron methyl |
|---|
| Class | Small Molecule |
|---|
| Description | Ethametsulfuron-methyl is a selective herbicide for the control of wild mustard, stinkweed and other broad-leaved weeds especially in canola. It is moderately soluble in water, non-volatile and, based on its chemical properties, presents a high risk of leaching to groundwater. It is relatively persistent in both soil and aqueous systems. It has a low mammalian toxicity but may bio-accumulate. It has a low toxicity to birds and most aquatic species, the exception being aquatic plants including algae. It is moderately toxic to honeybees |
|---|
| Compound Type | - Amide
- Ester
- Ether
- Herbicide
- Organic Compound
- Pesticide
- Synthetic Compound
|
|---|
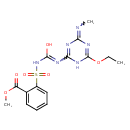
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Benzoic acid, 2-[[[[[4-ethoxy-6-(methylamino)-1,3,5-triazin-2-yl]amino]carbonyl]amino]sulfonyl]-, methyl ester | | ETHAMETSULFURON METHYL ESTER | | Ethametsulfuron methyl, Methyl 2-((4-ethoxy-6-methylamino-1,3,5-triazin-2-yl)carbamoylsulfamoyl)benzoate |
|
|---|
| Chemical Formula | C15H18N6O6S |
|---|
| Average Molecular Mass | 410.405 g/mol |
|---|
| Monoisotopic Mass | 410.101 g/mol |
|---|
| CAS Registry Number | 97780-06-8 |
|---|
| IUPAC Name | methyl 2-[({[6-ethoxy-4-(methylimino)-1,4-dihydro-1,3,5-triazin-2-yl]-C-hydroxycarbonimidoyl}amino)sulfonyl]benzoate |
|---|
| Traditional Name | methyl 2-({[4-ethoxy-6-(methylimino)-3H-1,3,5-triazin-2-yl]-C-hydroxycarbonimidoyl}aminosulfonyl)benzoate |
|---|
| SMILES | CCOC1=NC(=NC)N=C(N1)N=C(O)NS(=O)(=O)C1=CC=CC=C1C(=O)OC |
|---|
| InChI Identifier | InChI=1S/C15H18N6O6S/c1-4-27-15-19-12(16-2)17-13(20-15)18-14(23)21-28(24,25)10-8-6-5-7-9(10)11(22)26-3/h5-8H,4H2,1-3H3,(H3,16,17,18,19,20,21,23) |
|---|
| InChI Key | InChIKey=ZINJLDJMHCUBIP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as s-triazinyl-2-sulfonylureas. These are aromatic heterocyclic compounds containing a s-triazine ring which is substituted with a urea at the ring 2-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Sulfonylureas |
|---|
| Direct Parent | S-triazinyl-2-sulfonylureas |
|---|
| Alternative Parents | |
|---|
| Substituents | - S-triazinyl-2-sulfonylurea
- Benzenesulfonamide
- Benzoate ester
- Benzenesulfonyl group
- Benzoic acid or derivatives
- Alkoxy-s-triazine
- Benzoyl
- Alkyl aryl ether
- Amino-1,3,5-triazine
- Aminotriazine
- Secondary aliphatic/aromatic amine
- N-aliphatic s-triazine
- Monocyclic benzene moiety
- 1,3,5-triazine
- Triazine
- Benzenoid
- Sulfonyl
- Organosulfonic acid or derivatives
- Organic sulfonic acid or derivatives
- Methyl ester
- Aminosulfonyl compound
- Heteroaromatic compound
- Carbonic acid derivative
- Carboxylic acid ester
- Amino acid or derivatives
- Secondary amine
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Ether
- Carboxylic acid derivative
- Azacycle
- Organosulfur compound
- Amine
- Hydrocarbon derivative
- Organic oxygen compound
- Organic oxide
- Carbonyl group
- Organopnictogen compound
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0v0r-1509000000-6315f5362281fa004cbe | 2021-09-24 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-11-04 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-04 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-04 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-04 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-04 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-11-04 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0932300000-3ba92e923df744559c2a | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-2920000000-91a68732d056e77e7c21 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014r-3900000000-8e72fc8a3b78251b538b | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-07vu-2669400000-94918620552c8e5123f5 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-2390000000-6d84098b5c046f0a6031 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03ka-9520000000-65f77d6cc1a667cf29fa | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01r2-0904300000-cdec82b37f2c12e022b4 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gba-0914000000-8e3700fb4459eec6ec79 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-2912000000-2bf3d0e899849757b543 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0bt9-0960800000-5037e06dabba736c2a94 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-1900000000-2a8c65d4a11adc6f27be | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-9634000000-97258290d694ced48927 | 2021-10-12 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 91756 |
|---|
| ChEMBL ID | CHEMBL1885280 |
|---|
| ChemSpider ID | 82854 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D4501.pdf |
|---|
| General References | Not Available |
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|