| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-08 02:38:23 UTC |
|---|
| Update Date | 2014-12-24 20:26:54 UTC |
|---|
| Accession Number | T3D4627 |
|---|
| Identification |
|---|
| Common Name | 7H-Dibenzo[c,g]carbazole |
|---|
| Class | Small Molecule |
|---|
| Description | 7H-Dibenzo[c,g]carbazole (DBC) is is a needle-like solid, a potent multispecies, multisite carcinogen present in the environment. The metabolic activation pathways of DBC are not completely known. It may irritate the eyes, nose, and throat. |
|---|
| Compound Type | - Cigarette Toxin
- Lachrymator
- Natural Compound
- Organic Compound
|
|---|
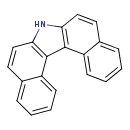
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C20H13N |
|---|
| Average Molecular Mass | 267.324 g/mol |
|---|
| Monoisotopic Mass | 267.105 g/mol |
|---|
| CAS Registry Number | 194-59-2 |
|---|
| IUPAC Name | 12-azapentacyclo[11.8.0.0²,¹¹.0³,⁸.0¹⁶,²¹]henicosa-1(13),2(11),3,5,7,9,14,16,18,20-decaene |
|---|
| Traditional Name | 7H-dibenzo(C,G)carbazole |
|---|
| SMILES | N1C2=C(C3=C1C=CC1=CC=CC=C31)C1=CC=CC=C1C=C2 |
|---|
| InChI Identifier | InChI=1S/C20H13N/c1-3-7-15-13(5-1)9-11-17-19(15)20-16-8-4-2-6-14(16)10-12-18(20)21-17/h1-12,21H |
|---|
| InChI Key | InChIKey=STJXCDGCXVZHDU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as carbazoles. Carbazoles are compounds containing a three ring system containing a pyrrole ring fused on either side to a benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Carbazoles |
|---|
| Direct Parent | Carbazoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Carbazole
- Naphthalene
- Indole
- Benzenoid
- Heteroaromatic compound
- Pyrrole
- Azacycle
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytosol
- Extracellular matrix
- Membrane
- Microsome
- Nucleolus
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Apoptosis | Not Available | map04210 |
|
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0090000000-16be8ce7e9408b10d329 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0090000000-09cf9358310074b41d58 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014l-0190000000-b1d066cabb479c08c0e0 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0090000000-8dd53e9ddc43622a8a5a | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0090000000-8dd53e9ddc43622a8a5a | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0090000000-ed1e9d1ae55f80fdc19c | 2016-08-03 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-014i-0690000000-ef23f53b46a489392c1a | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 22.53 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 2B, possibly carcinogenic to humans. (1) |
|---|
| Uses/Sources | This is a toxic chemical found in cigarettes or generated by tobacco combustion. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 9134 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 8780 |
|---|
| KEGG ID | C19221 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D4627.pdf |
|---|
| General References | - International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|