| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-08 02:41:29 UTC |
|---|
| Update Date | 2014-12-24 20:26:54 UTC |
|---|
| Accession Number | T3D4658 |
|---|
| Identification |
|---|
| Common Name | Dibenz(b,F)-1,4-oxazepine |
|---|
| Class | Small Molecule |
|---|
| Description | Dibenz(b,F)-1,4-oxazepine, also known as CR gas and dibenzoxazepine, is an incapacitating agent and a lachrymatory agent. It was developed by the British Ministry of Defence as a riot control agent in the late 1950s and early 1960s. Dibenz(b,F)-1,4-oxazepine is a lachrymatory agent (LA), exerting its effects through activation of the TRPA1 channel. It is a suspected carcinogen. It is toxic, but less so than CS gas (2-chlorobenzalmalononitrile), by ingestion and exposure. However, it can be lethal in large quantities. In a poorly ventilated space, an individual may inhale a lethal dose within minutes. Death is caused by asphyxiation and pulmonary edema. The effect of CR is long-term and persistent. CR can persist on surfaces, especially porous ones, for up to 60 days. |
|---|
| Compound Type | - Ether
- Industrial/Workplace Toxin
- Lachrymator
- Organic Compound
- Synthetic Compound
|
|---|
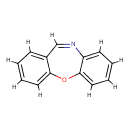
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Dibenzo[b,F][1,4]Oxazepine |
|
|---|
| Chemical Formula | C13H9NO |
|---|
| Average Molecular Mass | 195.217 g/mol |
|---|
| Monoisotopic Mass | 195.068 g/mol |
|---|
| CAS Registry Number | 257-07-8 |
|---|
| IUPAC Name | 2-oxa-9-azatricyclo[9.4.0.0³,⁸]pentadeca-1(15),3,5,7,9,11,13-heptaene |
|---|
| Traditional Name | dibenz(b,f)(1,4)oxazepine |
|---|
| SMILES | O1C2=CC=CC=C2C=NC2=CC=CC=C12 |
|---|
| InChI Identifier | InChI=1S/C13H9NO/c1-3-7-12-10(5-1)9-14-11-6-2-4-8-13(11)15-12/h1-9H |
|---|
| InChI Key | InChIKey=NPUACKRELIJTFM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dibenzoxazepines. Dibenzoxazepines are compounds containing a dibenzoxazepine moiety, which consists of two benzene connected by an oxazepine ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzoxazepines |
|---|
| Sub Class | Dibenzoxazepines |
|---|
| Direct Parent | Dibenzoxazepines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dibenzoxazepine
- Diaryl ether
- Benzenoid
- Ether
- Oxacycle
- Azacycle
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Imine
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | Pale yellow crystalline solid |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 73°C | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014j-0900000000-f32410c76b2c66da0096 | 2021-09-23 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-574736603d41da76ac7e | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0900000000-0d54063e0b78113d6f20 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004l-9000000000-f1043bb6e710e8f8aec1 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-b5ac83732cc40dd778a0 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0900000000-c25fd4ff43540c7aff73 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-0900000000-20f9b8c4e33802c0619b | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-0310cae5b7a4706b2038 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0900000000-0310cae5b7a4706b2038 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-0900000000-b394949869914211006c | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-fe1bdc84baadae0a8c3d | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0900000000-fe1bdc84baadae0a8c3d | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-0900000000-c42ddd885d7c716b0a8f | 2021-10-12 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not listed by IARC. |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 9213 |
|---|
| ChEMBL ID | CHEMBL1085100 |
|---|
| ChemSpider ID | 8858 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | CR_gas |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D4658.pdf |
|---|
| General References | - Brone B, Peeters PJ, Marrannes R, Mercken M, Nuydens R, Meert T, Gijsen HJ: Tear gasses CN, CR, and CS are potent activators of the human TRPA1 receptor. Toxicol Appl Pharmacol. 2008 Sep 1;231(2):150-6. doi: 10.1016/j.taap.2008.04.005. Epub 2008 Apr 20. [18501939 ]
- Gijsen HJ, Berthelot D, Zaja M, Brone B, Geuens I, Mercken M: Analogues of morphanthridine and the tear gas dibenz[b,f][1,4]oxazepine (CR) as extremely potent activators of the human transient receptor potential ankyrin 1 (TRPA1) channel. J Med Chem. 2010 Oct 14;53(19):7011-20. doi: 10.1021/jm100477n. [20806939 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|