| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-11 05:14:23 UTC |
|---|
| Update Date | 2014-12-24 20:26:56 UTC |
|---|
| Accession Number | T3D4743 |
|---|
| Identification |
|---|
| Common Name | Ethinyl Estradiol |
|---|
| Class | Small Molecule |
|---|
| Description | A semisynthetic alkylated estradiol with a 17-alpha-ethinyl substitution. It has high estrogenic potency when administered orally and is often used as the estrogenic component in oral contraceptives . Ethinyl estradiol is marketed mostly as a combination oral contraceptive under several brand names such as Alesse, Tri-Cyclen, Triphasil, and Yasmin. The FDA label includes a black box warning that states that combination oral contraceptives should not be used in women over 35 years old who smoke due to the increased risk of serious cardiovascular side effects. |
|---|
| Compound Type | - Drug
- Estrogen
- Food Toxin
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
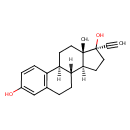
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 17 alpha-Ethinylestradiol | | 17 alpha-Ethynylestradiol | | 17 alpha-Ethynyloestradiol | | 17-Ethinyl-3,17-estradiol | | 17-Ethinyl-3,17-oestradiol | | 17-Ethinylestradiol | | 17-Ethynylestradiol | | 17-Ethynylestradiol ram | | 17-Ethynyloestradiol | | 17a-Ethinyl-17b-estradiol | | 17a-Ethinylestradiol | | 17a-Ethynyl-17b-oestradiol | | 17a-Ethynylestradiol-l7b | | 17a-Ethynyloestradiol | | 17a-Ethynyloestradiol-17b | | 17alpha-Ethinyl estradiol | | 17α-ethynylestradiol | | Alora | | Amenoron | | Anovlar | | Chee-O-Gen | | Chee-O-Genf | | Diognat-E | | Diogyn E | | Diogyn-E | | Dyloform | | Ertonyl | | Esclim | | Esteed | | Estigyn | | Estinyl | | Eston-E | | Estoral | | Estoral {[Orion]} | | Estorals | | Estring | | Estrogen | | Ethidol | | Ethinoral | | Ethinyl estradiol | | Ethinyl-Oestranol | | Ethinylestradiol | | Ethinylestradiolum | | Ethinylestriol | | Ethinyloestradiol | | Ethynyl estradiol | | Ethynylestradiol | | Ethynyloestradiol | | Eticyclin | | Eticyclol | | Eticylol | | Etinestrol | | Etinestryl | | Etinilestradiol | | Etinoestryl | | Etistradiol | | Etivex | | Feminone | | Fempatch | | Follicoral | | Ginestrene | | Gynodiol | | Gynolett | | Inestra | | Innofem | | Kolpolyn | | Linoral | | Lynoral | | Menolyn | | Menostar | | Neo-Estrone | | Nogest-S | | Novestrol | | Oradiol | | Orestralyn | | Orestrayln | | Ovex | | Palonyl | | Perovex | | Primogyn | | Primogyn C | | Primogyn M | | Progynon C | | Progynon M | | Spanestrin | | Thiuram E | | Thiuranide | | Vagifem | | Ylestrol |
|
|---|
| Chemical Formula | C20H24O2 |
|---|
| Average Molecular Mass | 296.403 g/mol |
|---|
| Monoisotopic Mass | 296.178 g/mol |
|---|
| CAS Registry Number | 57-63-6 |
|---|
| IUPAC Name | (1S,10R,11S,14R,15S)-14-ethynyl-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-triene-5,14-diol |
|---|
| Traditional Name | (1S,10R,11S,14R,15S)-14-ethynyl-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-triene-5,14-diol |
|---|
| SMILES | [H][C@]12CC[C@](O)(C#C)[C@]1(C)CC[C@@]1([H])C3=CC=C(O)C=C3CC[C@]21[H] |
|---|
| InChI Identifier | InChI=1/C20H24O2/c1-3-20(22)11-9-18-17-6-4-13-12-14(21)5-7-15(13)16(17)8-10-19(18,20)2/h1,5,7,12,16-18,21-22H,4,6,8-11H2,2H3/t16-,17-,18+,19+,20+/s2 |
|---|
| InChI Key | InChIKey=BFPYWIDHMRZLRN-RHKZOZTBNA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as estrogens and derivatives. These are steroids with a structure containing a 3-hydroxylated estrane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Estrane steroids |
|---|
| Direct Parent | Estrogens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Estrogen-skeleton
- 3-hydroxysteroid
- Hydroxysteroid
- 17-hydroxysteroid
- Phenanthrene
- Tetralin
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Ynone
- Cyclic alcohol
- Tertiary alcohol
- Acetylide
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Alcohol
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | - Adipose Tissue
- Adrenal Cortex
- Adrenal Gland
- Bladder
- Brain
- Epidermis
- Fibroblasts
- Gonads
- Intestine
- Kidney
- Liver
- Muscle
- Nerve Cells
- Neuron
- Pancreas
- Placenta
- Platelet
- Prostate
- Skeletal Muscle
- Spleen
- Stratum Corneum
- Testes
- Thyroid Gland
|
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 142-144°C | | Boiling Point | Not Available | | Solubility | 11.3 mg/L (at 27°C) | | LogP | 3.67 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-03di-0890000000-5219bc8ac1212313c9c0 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-03di-0890000000-5219bc8ac1212313c9c0 | 2018-05-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-06ec-0890000000-23c55dc07e698aa701e4 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01t9-3264900000-27be1780fbc6934a75b0 | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-000b-0290000000-0ef72fb5e00440b9ff50 | 2012-07-25 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-000i-0960000000-945d82a0ab94f246ac88 | 2012-07-25 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-056s-2910000000-0d38daebb87a39304e91 | 2012-07-25 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-80) , Positive | splash10-03di-0890000000-7a166e952207cf7bc1d3 | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0a4i-1790000000-194c2e946cdaab2072ce | 2021-09-20 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0190000000-075ad04641619da4b2f5 | 2017-07-26 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004j-0390000000-70fa13954d5025b402ba | 2017-07-26 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fbc-6980000000-8d3156c6218c2f54df34 | 2017-07-26 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-6490bc65476d939667d6 | 2017-07-26 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-9c8e8277a610eab839c8 | 2017-07-26 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00p0-0090000000-87d77005eb0529ab1abb | 2017-07-26 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-fb34058a34b84424b009 | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fba-0890000000-310cbc77999e8d6b02f9 | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0295-2940000000-a936403339b6f3803c0c | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-ebc6f53e480399549577 | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-73e320051b256d09bbb0 | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014r-0090000000-4c31cf3a8784d6c604f1 | 2021-09-25 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-03dj-2980000000-831c8190c59f98fc8623 | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, CDCl3, experimental) | Not Available | 2012-12-05 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 25.16 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CDCl3, experimental) | Not Available | 2012-12-05 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Rapid and complete absorption follows oral intake of ethinyl estradiol (bioavailability 43%). |
|---|
| Mechanism of Toxicity | Estrogens diffuse into their target cells and interact with a protein receptor. Target cells include the female reproductive tract, the mammary gland, the hypothalamus, and the pituitary. Estrogens increase the hepatic synthesis of sex hormone binding globulin (SHBG), thyroid-binding globulin (TBG), and other serum proteins and suppress follicle-stimulating hormone (FSH) from the anterior pituitary. This cascade is initiated by initially binding to the estrogen receptors. The combination of an estrogen with a progestin suppresses the hypothalamic-pituitary system, decreasing the secretion of gonadotropin-releasing hormone (GnRH). |
|---|
| Metabolism | Hepatic. Quantitatively, the major metabolic pathway for ethinyl estradiol, both in rats and in humans, is aromatic hydroxylation, as it is for the natural estrogens.
Half Life: 36 +/- 13 hours |
|---|
| Toxicity Values | Oral, mouse LD50: 1737 mg/kg. |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For treatment of moderate to severe vasomotor symptoms associated with the menopause, female hypogonadism, prostatic carcinoma-palliative therapy of advanced disease, breast cancer, as an oral contraceptive, and as emergency contraceptive. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Symptoms of overdose include nausea and vomiting, and withdrawal bleeding may occur in females. The FDA label includes a black box warning that states that combination oral contraceptives with ethinyl estradiol should not be used in women over 35 years old who smoke due to the increased risk of serious cardiovascular side effects. |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00977 |
|---|
| HMDB ID | HMDB01926 |
|---|
| PubChem Compound ID | 5991 |
|---|
| ChEMBL ID | CHEMBL691 |
|---|
| ChemSpider ID | 5770 |
|---|
| KEGG ID | C07534 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 4903 |
|---|
| BioCyc ID | ABIETADIENE |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Ethinylestradiol |
|---|
| References |
|---|
| Synthesis Reference | Andreas Sachse, “Method of female hormonal contraception using a fixed extended cycle hormonal preparation containing dienogest and ethinyl estradiol.” U.S. Patent US20060079491, issued April 13, 2006. |
|---|
| MSDS | Link |
|---|
| General References | - Li X, Strauss L, Kaatrasalo A, Mayerhofer A, Huhtaniemi I, Santti R, Makela S, Poutanen M: Transgenic mice expressing p450 aromatase as a model for male infertility associated with chronic inflammation in the testis. Endocrinology. 2006 Mar;147(3):1271-7. Epub 2005 Nov 23. [16306085 ]
- Wernert N: [Immunohistochemistry of the prostate and prostate carcinomas]. Veroff Pathol. 1991;135:1-163. [2038892 ]
- Kaufer D, Ogle WO, Pincus ZS, Clark KL, Nicholas AC, Dinkel KM, Dumas TC, Ferguson D, Lee AL, Winters MA, Sapolsky RM: Restructuring the neuronal stress response with anti-glucocorticoid gene delivery. Nat Neurosci. 2004 Sep;7(9):947-53. Epub 2004 Aug 8. [15300253 ]
- Zhang L, Sukhareva M, Barker JL, Maric D, Hao Y, Chang YH, Ma W, O'Shaughnessy T, Rubinow DR: Direct binding of estradiol enhances Slack (sequence like a calcium-activated potassium channel) channels' activity. Neuroscience. 2005;131(2):275-82. [15708472 ]
- Herrero P, Soto PF, Dence CS, Kisrieva-Ware Z, Delano DA, Peterson LR, Gropler RJ: Impact of hormone replacement on myocardial fatty acid metabolism: potential role of estrogen. J Nucl Cardiol. 2005 Sep-Oct;12(5):574-81. [16171718 ]
- Shah MG, Maibach HI: Estrogen and skin. An overview. Am J Clin Dermatol. 2001;2(3):143-50. [11705091 ]
- Safi R, Kovacic A, Gaillard S, Murata Y, Simpson ER, McDonnell DP, Clyne CD: Coactivation of liver receptor homologue-1 by peroxisome proliferator-activated receptor gamma coactivator-1alpha on aromatase promoter II and its inhibition by activated retinoid X receptor suggest a novel target for breast-specific antiestrogen therapy. Cancer Res. 2005 Dec 15;65(24):11762-70. [16357189 ]
- vom Saal FS, Grant WM, McMullen CW, Laves KS: High fetal estrogen concentrations: correlation with increased adult sexual activity and decreased aggression in male mice. Science. 1983 Jun 17;220(4603):1306-9. [6857252 ]
- Schmitt PM, Gohil K, Kaufman MP: Spinal estrogen attenuates the exercise pressor reflex but has little effect on the expression of genes regulating neurotransmitters in the dorsal root ganglia. J Appl Physiol (1985). 2006 Mar;100(3):958-64. Epub 2005 Nov 23. [16306253 ]
- Kalaitzidis D, Ok J, Sulak L 2nd, Starczynowski DT, Gilmore TD: Characterization of a human REL-estrogen receptor fusion protein with a reverse conditional transforming activity in chicken spleen cells. Oncogene. 2004 Sep 30;23(45):7580-7. [15326488 ]
- Pierard-Franchimont C, Letawe C, Goffin V, Pierard GE: Skin water-holding capacity and transdermal estrogen therapy for menopause: a pilot study. Maturitas. 1995 Sep;22(2):151-4. [8538484 ]
- de la Monte SM, Moore GW, Hutchins GM: Metastatic behavior of prostate cancer. Cluster analysis of patterns with respect to estrogen treatment. Cancer. 1986 Aug 15;58(4):985-93. [3719562 ]
- Czernik PJ, Little JM, Barone GW, Raufman JP, Radominska-Pandya A: Glucuronidation of estrogens and retinoic acid and expression of UDP-glucuronosyltransferase 2B7 in human intestinal mucosa. Drug Metab Dispos. 2000 Oct;28(10):1210-6. [10997942 ]
- Harris H, Henderson R, Bhat R, Komm B: Regulation of metallothionein II messenger ribonucleic acid measures exogenous estrogen receptor-beta activity in SAOS-2 and LNCaPLN3 cells. Endocrinology. 2001 Feb;142(2):645-52. [11159835 ]
- Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, Christie N, Finkelstein S, Siegfried JM: Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002 Apr 1;62(7):2141-50. [11929836 ]
- Frolich A, Christensen L, Andersen J: Estrogen receptors appear undetectable in the C-cells of the human thyroid gland. Bone. 1990;11(6):393-6. [1706609 ]
- Siiteri PK: Review of studies on estrogen biosynthesis in the human. Cancer Res. 1982 Aug;42(8 Suppl):3269s-3273s. [7083184 ]
- Koehler KF, Helguero LA, Haldosen LA, Warner M, Gustafsson JA: Reflections on the discovery and significance of estrogen receptor beta. Endocr Rev. 2005 May;26(3):465-78. Epub 2005 Apr 27. [15857973 ]
- Moro L, Reineri S, Piranda D, Pietrapiana D, Lova P, Bertoni A, Graziani A, Defilippi P, Canobbio I, Torti M, Sinigaglia F: Nongenomic effects of 17beta-estradiol in human platelets: potentiation of thrombin-induced aggregation through estrogen receptor beta and Src kinase. Blood. 2005 Jan 1;105(1):115-21. Epub 2004 Jun 15. [15198955 ]
- Nakano Y, Oshima T, Ozono R, Ueda A, Oue Y, Matsuura H, Sanada M, Ohama K, Chayama K, Kambe M: Estrogen replacement suppresses function of thrombin stimulated platelets by inhibiting Ca(2+) influx and raising cyclic adenosine monophosphate. Cardiovasc Res. 2002 Feb 15;53(3):634-41. [11861034 ]
- FDA label.
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|