| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-11 05:14:42 UTC |
|---|
| Update Date | 2014-12-24 20:26:56 UTC |
|---|
| Accession Number | T3D4749 |
|---|
| Identification |
|---|
| Common Name | Levonorgestrel |
|---|
| Class | Small Molecule |
|---|
| Description | A synthetic progestational hormone with actions similar to those of progesterone and about twice as potent as its racemic or (+-)-isomer (norgestrel). It is used for contraception, control of menstrual disorders, and treatment of endometriosis. It is usually supplied in a racemic mixture (Norgestrel, 6533-00-2). Only the levonorgestrel isomer is active. Levonorgestrel is marketed mostly as a combination oral contraceptive under several brand names such as Alesse, Triphasil, and Min-Ovral. |
|---|
| Compound Type | - Contraceptive Agent
- Contraceptive Agent, Female
- Contraceptive, Oral, Synthetic
- Drug
- Ester
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
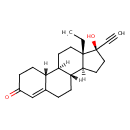
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (-)-13-Ethyl-17-hydroxy-18,19-dinor-17alpha-pregn-4-en-20-yn-3-one | | (8R,9S,10R,13S,14S,17R)-13-ethyl-17-ethynyl-17-hydroxy- 1,2,6,7,8,9,10,11,12,13,14,15,16, 17- tetradecahydrocyclopenta[a] phenanthren-3-one | | 13-beta-Ethyl-17alpha-ethynyl-17beta-hydroxygon-4-en-3-one | | 13-Ethyl-17-alpha-ethynyl-17-beta-hydroxy-4-gonen-3-one | | 13-Ethyl-17-alpha-ethynylgon-4-en-17-beta-ol-3-one | | 17-alpha-Ethinyl-13-beta-ethyl-17-beta-hydroxy-4-estren-3-one | | 17-alpha-Ethynyl-13-ethyl-19-nortestosterone | | 17-Ethynyl-18-methyl-19-nortestosterone | | 17alpha-Ethynyl-13beta-ethyl-3-oxo-4-estren-17beta-ol | | 17alpha-Ethynyl-17-hydroxy-18-methylestr-4-en-3-one | | 17alpha-Ethynyl-18-homo-19-nortestosterone | | 18-Methyl-17-alpha-ethynyl-19-nortestosterone | | 18-Methylnorethisterone | | D(-)-Norgestrel | | Jadelle | | Levonelle | | Levonorgestrelum | | Levonova | | Medonor | | Microlut | | Microluton | | Microval | | Mirena | | Neogest | | Next Choice | | Norgeston | | NorLevo | | Norplant | | Plan b | | Postinor | | Skyla |
|
|---|
| Chemical Formula | C21H28O2 |
|---|
| Average Molecular Mass | 312.446 g/mol |
|---|
| Monoisotopic Mass | 312.209 g/mol |
|---|
| CAS Registry Number | 797-63-7 |
|---|
| IUPAC Name | (1S,2R,10R,11S,14R,15S)-15-ethyl-14-ethynyl-14-hydroxytetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one |
|---|
| Traditional Name | (1S,2R,10R,11S,14R,15S)-15-ethyl-14-ethynyl-14-hydroxytetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one |
|---|
| SMILES | [H][C@@]12CC[C@@](O)(C#C)[C@@]1(CC)CC[C@]1([H])[C@@]3([H])CCC(=O)C=C3CC[C@@]21[H] |
|---|
| InChI Identifier | InChI=1S/C21H28O2/c1-3-20-11-9-17-16-8-6-15(22)13-14(16)5-7-18(17)19(20)10-12-21(20,23)4-2/h2,13,16-19,23H,3,5-12H2,1H3/t16-,17+,18+,19-,20-,21-/m0/s1 |
|---|
| InChI Key | InChIKey=WWYNJERNGUHSAO-XUDSTZEESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as estrogens and derivatives. These are steroids with a structure containing a 3-hydroxylated estrane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Estrane steroids |
|---|
| Direct Parent | Estrogens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Estrogen-skeleton
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- 17-hydroxysteroid
- Oxosteroid
- Hydroxysteroid
- Delta-4-steroid
- Cyclohexenone
- Ynone
- Cyclic alcohol
- Tertiary alcohol
- Cyclic ketone
- Ketone
- Acetylide
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Alcohol
- Organooxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | - terminal acetylenic compound (CHEBI:6443 )
- 3-oxo Delta(4)-steroid (CHEBI:6443 )
- 17beta-hydroxy steroid (CHEBI:6443 )
- Pregnane and derivatives [Fig] (C08153 )
- C21 steroids (gluco/mineralocorticoids, progestogens) and derivatives (C08153 )
- C21 steroids (gluco/mineralocorticoids, progestogins) and derivatives (LMST02030119 )
|
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 240°C | | Boiling Point | Not Available | | Solubility | 2.05 mg/L | | LogP | 3.8 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-0490000000-497c4ebd506c0b9c8ec3 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0a4l-1259000000-2a7acf9f8fd01faadc86 | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-066r-0931000000-4a863e895a5edd6cebeb | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-03di-0439000000-bcbc7c657a115dced40a | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0a5a-3920000000-edbd3785e7d20d9e81be | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0a4i-2920000000-a08b14b6d762cf4c42b1 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Negative | splash10-0002-0900000000-ccbe00eb6d33f44bf13e | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-03dj-0809000000-3b4f03aa1582c55a88a4 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-0a59-2910000000-2e210ea979850c3d19ac | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Negative | splash10-0002-0900000000-87a5333787ffe2461b52 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-03dj-1943000000-e4eeb86036aa8456827f | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-06r2-0932000000-542d94c4f65730336be8 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Negative | splash10-0002-0900000000-d9c9cc75f7d652292590 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Negative | splash10-0002-0900000000-a5a5bd10465f22e61a65 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-03di-0009000000-0d184562b2bcf62fab5c | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-055f-7900000000-2c2242a1883d4b23fb14 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-0a5c-4900000000-64d2115a1e9554b15780 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-05po-9700000000-f172af0f505e7230dfcc | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Negative | splash10-03di-0009000000-73765d8e0531c2c94251 | 2021-09-20 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0179000000-09f92950350c9411f4dc | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0btj-0191000000-7bd7256abde150b22d9f | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f76-2390000000-84a923da67ade6ef90a8 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0019000000-3f0cb0e2e76214741c6f | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0059000000-f6e5c83ac4c0da584cb2 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-053v-0090000000-6bb0ea8f0fc81b4c6fe2 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-152b659b4891e36b54f3 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0019000000-51b6ec5de157050ca35e | 2021-09-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 50.18 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Levonorgestrel is not subjected to a "first-pass" effect and is virtually 100% bioavailable. |
|---|
| Mechanism of Toxicity | Binds to the progesterone and estrogen receptors. Target cells include the female reproductive tract, the mammary gland, the hypothalamus, and the pituitary. Once bound to the receptor, progestins like levonorgestrel will slow the frequency of release of gonadotropin releasing hormone (GnRH) from the hypothalamus and blunt the pre-ovulatory LH (luteinizing hormone) surge. |
|---|
| Metabolism | Hepatic.
Route of Elimination: About 45% of levonorgestrel and its metabolites are excreted in the urine and about 32% are excreted in feces, mostly as glucuronide conjugates. |
|---|
| Toxicity Values | LD50 >5000 mg/kg (orally in rats) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Norgestrel: Group 2B, possibly carcinogenic to humans. (4) |
|---|
| Uses/Sources | For the treatment of menopausal and postmenopausal disorders and alone or in combination with other hormones as an oral contraceptive. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00367 |
|---|
| HMDB ID | HMDB14511 |
|---|
| PubChem Compound ID | 13109 |
|---|
| ChEMBL ID | CHEMBL1389 |
|---|
| ChemSpider ID | 12560 |
|---|
| KEGG ID | C08149 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 6443 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | NOG |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Levonorgestrel |
|---|
| References |
|---|
| Synthesis Reference | Yu-Sheng Chang, Shu-Ping Chen, “Levonorgestrel Crystallization.” U.S. Patent US20090069584, issued March 12, 2009. |
|---|
| MSDS | Link |
|---|
| General References | - Edgren RA, Stanczyk FZ: Nomenclature of the gonane progestins. Contraception. 1999 Dec;60(6):313. [10715364 ]
- Sitruk-Ware R: New progestagens for contraceptive use. Hum Reprod Update. 2006 Mar-Apr;12(2):169-78. Epub 2005 Nov 16. [16291771 ]
- FDA label.
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|