| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-11 05:16:19 UTC |
|---|
| Update Date | 2014-12-24 20:26:57 UTC |
|---|
| Accession Number | T3D4781 |
|---|
| Identification |
|---|
| Common Name | Hydrochlorothiazide |
|---|
| Class | Small Molecule |
|---|
| Description | A thiazide diuretic often considered the prototypical member of this class. It reduces the reabsorption of electrolytes from the renal tubules. This results in increased excretion of water and electrolytes, including sodium, potassium, chloride, and magnesium. It has been used in the treatment of several disorders including edema, hypertension, diabetes insipidus, and hypoparathyroidism. |
|---|
| Compound Type | - Amide
- Amine
- Antihypertensive Agent
- Diuretic
- Drug
- Food Toxin
- Metabolite
- Organic Compound
- Organochloride
- Sodium Chloride Symporter Inhibitor
- Synthetic Compound
|
|---|
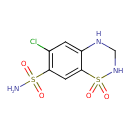
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 3,4-Dihydrochlorothiazide | | Acuretic | | Aldactazide | | Aldoril | | Apo-hydro | | Apresazide | | Aquarills | | Aquarius | | Bremil | | Caplaril | | Capozide | | Chlorosulthiadil | | Chlorothiazide | | Chlorsulfonamidodihydrobenzothiadiazine dioxide | | Chlorzide | | Cidrex | | Dichlorosal | | Dichlotiazid | | Dichlotride | | Diclotride | | Dihydrochlorothiazid | | Dihydrochlorothiazide | | Dihydrochlorothiazidum | | Dihydrochlorurit | | Dihydrochlorurite | | Dihydroxychlorothiazidum | | Direma | | Disalunil | | Diuril | | Drenol | | Dyazide | | Esidrex | | Esidrix | | Esimil | | Fluvin | | HCTZ | | HCZ | | Hidril | | Hidrochlortiazid | | Hidroronol | | Hidrotiazida | | Hydril | | Hydro-Aquil | | Hydro-D | | Hydro-Diuril | | Hydrochloro Thiazide | | Hydrochlorothiazid | | Hydrochlorothiazide Intensol | | Hydrochlorthiazide | | Hydrodiuretic | | Hydrodiuril | | Hydropres | | Hydrosaluric | | Hydrothide | | Hydrozide | | Hypothiazid | | Hypothiazide | | Hyzaar | | Idrotiazide | | Inderide | | Ivaugan | | Jen-Diril | | Lopressor HCT | | Lotensin HCT | | Maschitt | | Maxzide | | Megadiuril | | Microzide | | Moduretic | | Nefrix | | Neo-codema | | Neoflumen | | Newtolide | | Oretic | | Panurin | | Prinzide | | Ro-hydrazide | | Ser-Ap-Es | | Servithiazid | | Thiaretic | | Thiuretic | | Thlaretic | | Timolide | | Unipres | | Urodiazin | | Vaseretic | | Vetidrex | | Ziac | | Zide |
|
|---|
| Chemical Formula | C7H8ClN3O4S2 |
|---|
| Average Molecular Mass | 297.739 g/mol |
|---|
| Monoisotopic Mass | 296.964 g/mol |
|---|
| CAS Registry Number | 58-93-5 |
|---|
| IUPAC Name | 6-chloro-1,1-dioxo-3,4-dihydro-2H-1lambda6,2,4-benzothiadiazine-7-sulfonamide |
|---|
| Traditional Name | 6-chloro-1,1-dioxo-3,4-dihydro-2H-1lambda6,2,4-benzothiadiazine-7-sulfonamide |
|---|
| SMILES | NS(=O)(=O)C1=CC2=C(NCNS2(=O)=O)C=C1Cl |
|---|
| InChI Identifier | InChI=1S/C7H8ClN3O4S2/c8-4-1-5-7(2-6(4)16(9,12)13)17(14,15)11-3-10-5/h1-2,10-11H,3H2,(H2,9,12,13) |
|---|
| InChI Key | InChIKey=JZUFKLXOESDKRF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,2,4-benzothiadiazine-1,1-dioxides. These are aromatic heterocyclic compounds containing a 1,2,4-benzothiadiazine ring system with two S=O bonds at the 1-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Thiadiazines |
|---|
| Sub Class | Benzothiadiazines |
|---|
| Direct Parent | 1,2,4-benzothiadiazine-1,1-dioxides |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,2,4-benzothiadiazine-1,1-dioxide
- Secondary aliphatic/aromatic amine
- Aryl chloride
- Aryl halide
- Organosulfonic acid amide
- Benzenoid
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Aminosulfonyl compound
- Sulfonyl
- Secondary amine
- Azacycle
- Organic oxide
- Amine
- Organopnictogen compound
- Organosulfur compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | - Adipose Tissue
- Kidney
- Platelet
|
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 266-268°C | | Boiling Point | Not Available | | Solubility | 722 mg/L (at 25°C) | | LogP | -0.07 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014i-1490000000-623736f34c52e89bbc88 | 2017-08-28 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-014i-0090000000-7906a6e4cfb51dce1396 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-0002-0090000000-c44dbed5084dc5df03d8 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-0002-0090000000-976dcb246a2272b22565 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-0uxr-1090000000-16e563e592e4a566424d | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-0fb9-9580000000-3c9227e3df8ed6dc9ae9 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-004i-9720000000-12525bfcb83ea1611970 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-004i-9200000000-df37b796b59f084b0229 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-0002-0090000000-220666bc28231ad387e8 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-0002-0090000000-f434c5d8ee94540ec0d2 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-0uxr-1090000000-8a3c270e0fb285b26f15 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-0fb9-9480000000-5f6c5ed1d291d71a0aae | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-004i-9520000000-679def33e8239c1382f3 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-004i-9400000000-c9b80274d62fda64c531 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-014i-0090000000-0a962453b9e31eec3822 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-0002-0090000000-ac2b5d07eaacba87a181 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-0gb9-1090000000-8649018477d708cb6bd7 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-004i-9400000000-266cd4efe3deeebe29f3 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-014i-0090000000-bc85dd2515151e4fec03 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Negative | splash10-004i-9200000000-0f74393a0813e692bd20 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Negative | splash10-004i-9300000000-28e5742a9d4eca7b8b56 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Negative | splash10-004i-9320000000-b939e4fba37f7da92d72 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Negative | splash10-0fb9-5190000000-04ff81457234f6f9b8e1 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Negative | splash10-004i-9200000000-df37b796b59f084b0229 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Negative | splash10-004i-9720000000-12525bfcb83ea1611970 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Negative | splash10-0002-0090000000-220666bc28231ad387e8 | 2021-09-20 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-00r2-6490000000-3f5d9320308b823c6489 | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | 2012-12-05 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, DMSO-d6, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100.40 MHz, DMSO-d6, experimental) | Not Available | 2014-09-23 | View Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | 2012-12-05 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | 50-60% |
|---|
| Mechanism of Toxicity | Hydrochlorothiazide, a thiazide diuretic, inhibits water reabsorption in the nephron by inhibiting the sodium-chloride symporter (SLC12A3) in the distal convoluted tubule, which is responsible for 5% of total sodium reabsorption. Normally, the sodium-chloride symporter transports sodium and chloride from the lumen into the epithelial cell lining the distal convoluted tubule. The energy for this is provided by a sodium gradient established by sodium-potassium ATPases on the basolateral membrane. Once sodium has entered the cell, it is transported out into the basolateral interstitium via the sodium-potassium ATPase, causing an increase in the osmolarity of the interstitium, thereby establishing an osmotic gradient for water reabsorption. By blocking the sodium-chloride symporter, hydrochlorothiazide effectively reduces the osmotic gradient and water reabsorption throughout the nephron. |
|---|
| Metabolism | Hydrochlorothiazide is not metabolized.
Route of Elimination: Hydrochlorothiazide is not metabolized but is eliminated rapidly by the kidney. Hydrochlorothiazide crosses the placental but not the blood-brain barrier and is excreted in breast milk.
Half Life: 5.6 and 14.8 hours |
|---|
| Toxicity Values | The oral LD50 of hydrochlorothiazide is greater than 10 g/kg in the mouse and rat. |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 2B, possibly carcinogenic to humans. (16) |
|---|
| Uses/Sources | For the treatment of high blood pressure and management of edema. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | The most common signs and symptoms observed are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias. |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00999 |
|---|
| HMDB ID | HMDB01928 |
|---|
| PubChem Compound ID | 3639 |
|---|
| ChEMBL ID | CHEMBL435 |
|---|
| ChemSpider ID | 3513 |
|---|
| KEGG ID | C07041 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 102045 |
|---|
| BioCyc ID | 12-DEHYDRORETICULINIUM |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | HCZ |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Hydrochlorothiazide |
|---|
| References |
|---|
| Synthesis Reference | Frederic J. Nugent, John K. C. Yen, “Process for preparing the combination products of triamterene and hydrochlorothiazide.” U.S. Patent US4804540, issued July, 1987. |
|---|
| MSDS | Link |
|---|
| General References | - Dimitriadis G, Tegos C, Golfinopoulou L, Roboti C, Raptis S: Furosemide-induced hyperglycaemia: the implication of glycolytic kinases. Horm Metab Res. 1993 Nov;25(11):557-9. [8288156 ]
- Vandenheuvel WJ, Gruber VF, Walker RW, Wolf FJ: GLC analysis of hydrochlorothiazide in blood and plasma. J Pharm Sci. 1975 Aug;64(8):1309-12. [1151702 ]
- Yamazaki M, Ito Y, Suzuka T, Yaginuma H, Itoh S, Kamada A, Orita Y, Nakahama H, Nakanishi T, Ando A: Biopharmaceutical studies of thiazide diuretics. II. High-performance liquid chromatographic method for determination of hydrochlorothiazide in plasma, urine, blood cells and bile. Chem Pharm Bull (Tokyo). 1984 Jun;32(6):2387-94. [6488407 ]
- Germano G, Sanguigni V, Pignatelli P, Caccese D, Lenti L, Ragazzo M, Lauro R, Violi F: Enhanced platelet release of superoxide anion in systemic hypertension: role of AT1 receptors. J Hypertens. 2004 Jun;22(6):1151-6. [15167450 ]
- Bernik MM, Heimann JC, Nakandakare ER, Cazita PM, Nunes VS, Rocha JC, Neves MQ, Quintao EC: Effects of hydrochlorothiazide and propranolol treatment on chylomicron metabolism in hypertensive objects. Can J Physiol Pharmacol. 2005 Jul;83(7):617-23. [16091787 ]
- Splendiani G, Condo S: [Diuretic therapy in heart failure]. G Ital Nefrol. 2006 Jan-Feb;23 Suppl 34:S74-6. [16634001 ]
- Dornhorst A, Powell SH, Pensky J: Aggravation by propranolol of hyperglycaemic effect of hydrochlorothiazide in type II diabetics without alteration of insulin secretion. Lancet. 1985 Jan 19;1(8421):123-6. [2857210 ]
- Serradeil-Le Gal C, Lacour C, Valette G, Garcia G, Foulon L, Galindo G, Bankir L, Pouzet B, Guillon G, Barberis C, Chicot D, Jard S, Vilain P, Garcia C, Marty E, Raufaste D, Brossard G, Nisato D, Maffrand JP, Le Fur G: Characterization of SR 121463A, a highly potent and selective, orally active vasopressin V2 receptor antagonist. J Clin Invest. 1996 Dec 15;98(12):2729-38. [8981918 ]
- Kuo BS, Mandagere A, Osborne DR, Hwang KK: Column-switching high-performance liquid chromatographic (HPLC) determination of hydrochlorothiazide in rat, dog, and human plasma. Pharm Res. 1990 Dec;7(12):1257-61. [2095563 ]
- Cubeddu LX, Hoffmann IS, Davila S, Escontrelas C, Morales C, Rios A: Effects of propranolol, clonidine and hydrochlorothiazide treatment and abrupt discontinuation on central and peripheral noradrenergic activity in essential hypertension. Life Sci. 1986 Dec 22;39(25):2463-74. [3540504 ]
- Angelin B: Effect of thiazide treatment on biliary lipid composition in healthy volunteers. Eur J Clin Pharmacol. 1989;37(1):95-6. [2591472 ]
- Buttar HS: An overview of the influence of ACE inhibitors on fetal-placental circulation and perinatal development. Mol Cell Biochem. 1997 Nov;176(1-2):61-71. [9406146 ]
- Tisdall PA, Moyer TP, Anhalt JP: Liquid-chromatographic detection of thiazide diuretics in urine. Clin Chem. 1980 May;26(6):702-6. [7371146 ]
- Beermann B, Fahraeus L, Groschinsky-Grind M, Lindstrom B: Placental transfer of hydrochlorothiazide. Gynecol Obstet Invest. 1980;11(1):45-8. [7390276 ]
- Vonaparti A, Kazanis M, Panderi I: Development and validation of a liquid chromatographic/electrospray ionization mass spectrometric method for the determination of benazepril, benazeprilat and hydrochlorothiazide in human plasma. J Mass Spectrom. 2006 May;41(5):593-605. [16541390 ]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|