| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-11 05:18:08 UTC |
|---|
| Update Date | 2014-12-24 20:26:57 UTC |
|---|
| Accession Number | T3D4825 |
|---|
| Identification |
|---|
| Common Name | Ilepatril |
|---|
| Class | Small Molecule |
|---|
| Description | Ilepatril is a dual angiotensin-converting enzyme and neutral endopeptidase inhibitor, for the treatment of hypertension and diabetic nephropathy. |
|---|
| Compound Type | |
|---|
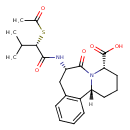
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C22H28N2O5S |
|---|
| Average Molecular Mass | 432.533 g/mol |
|---|
| Monoisotopic Mass | 432.172 g/mol |
|---|
| CAS Registry Number | 473289-62-2 |
|---|
| IUPAC Name | (2R,6S,9S)-9-[(2S)-2-(acetylsulfanyl)-3-methylbutanamido]-8-oxo-7-azatricyclo[9.4.0.0²,⁷]pentadeca-1(11),12,14-triene-6-carboxylic acid |
|---|
| Traditional Name | ilepatril |
|---|
| SMILES | [H][C@]12CCC[C@H](N1C(=O)[C@H](CC1=C2C=CC=C1)NC(=O)[C@@H](SC(C)=O)C(C)C)C(O)=O |
|---|
| InChI Identifier | InChI=1/C22H28N2O5S/c1-12(2)19(30-13(3)25)20(26)23-16-11-14-7-4-5-8-15(14)17-9-6-10-18(22(28)29)24(17)21(16)27/h4-5,7-8,12,16-19H,6,9-11H2,1-3H3,(H,23,26)(H,28,29)/t16-,17+,18-,19-/s2 |
|---|
| InChI Key | InChIKey=FXKFFTMLFPWYFH-DPYDJAPQNA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Dipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-dipeptide
- N-acyl-alpha amino acid or derivatives
- Benzazepine
- Alpha-amino acid or derivatives
- Piperidinecarboxylic acid
- Azepine
- Fatty amide
- N-acyl-amine
- Fatty acyl
- Piperidine
- Benzenoid
- Tertiary carboxylic acid amide
- Carboxamide group
- Lactam
- Carbothioic s-ester
- Secondary carboxylic acid amide
- Thiocarboxylic acid ester
- Organoheterocyclic compound
- Azacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Sulfenyl compound
- Thiocarboxylic acid or derivatives
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00el-1159500000-da9a818fddc8c0a305a2 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0191000000-01f04b51e6600fc7a24b | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-9371000000-81fe81fbeaf6110097f0 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-1309300000-68ac639f93a78ecff89e | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0083-2229200000-bce60cda30a86ee956cc | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-9821000000-d2ef0787e8aa01b669d6 | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB06604 |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | Not Available |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|