| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-11 05:19:54 UTC |
|---|
| Update Date | 2014-12-24 20:26:58 UTC |
|---|
| Accession Number | T3D4865 |
|---|
| Identification |
|---|
| Common Name | Sodium dehydroacetate |
|---|
| Class | Small Molecule |
|---|
| Description | Sodium dehydroacetate is used as a preservative for cut or peeled squash. |
|---|
| Compound Type | - Ester
- Food Additive
- Food Toxin
- Household Toxin
- Metabolite
- Organic Compound
- Plant Toxin
- Preservative
- Synthetic Compound
|
|---|
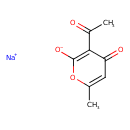
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 2H-Pyran-2,4(3H)-dione, 3-acetyl-6-methyl-, ion(1-), sodium | | 2H-Pyran-2,4(3H)-dione, 3-acetyl-6-methyl-, monosodium salt | | 2H-Pyran-2,4(3H)-dione, 3-acetyl-6-methyl-, sodium salt | | 3-Acetyl-6-methyl-2H-pyran-2,4(3H)-dione sodium salt | | Dehydroacetic acid, sodium salt | | Dha-S | | Dha-sodium | | DHN | | Harven | | New Side S 01 | | Prevan | | Sodium 3-acetyl-6-methyl-2,4-pyrandione | | Sodium 3-acetyl-6-methyl-2H-pyran-2,4(3H)-dione | | Sodium dehydroacetate (NF) | | Sodium dehydroacetic acid |
|
|---|
| Chemical Formula | C8H7NaO4 |
|---|
| Average Molecular Mass | 190.129 g/mol |
|---|
| Monoisotopic Mass | 190.024 g/mol |
|---|
| CAS Registry Number | 4418-26-2 |
|---|
| IUPAC Name | sodium 3-acetyl-6-methyl-4-oxo-4H-pyran-2-olate |
|---|
| Traditional Name | sodium 3-acetyl-6-methyl-4-oxopyran-2-olate |
|---|
| SMILES | [Na+].CC(=O)C1=C([O-])OC(C)=CC1=O |
|---|
| InChI Identifier | InChI=1S/C8H8O4.Na/c1-4-3-6(10)7(5(2)9)8(11)12-4;/h3,11H,1-2H3;/q;+1/p-1 |
|---|
| InChI Key | InChIKey=ISZAPZSICMMUCY-UHFFFAOYSA-M |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aryl alkyl ketones. These are ketones have the generic structure RC(=O)R', where R = aryl group and R'=alkyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Aryl alkyl ketones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aryl alkyl ketone
- Pyranone
- Pyran
- Heteroaromatic compound
- Vinylogous acid
- Cyclic ketone
- Oxacycle
- Organic alkali metal salt
- Organoheterocyclic compound
- Organic oxide
- Hydrocarbon derivative
- Organic sodium salt
- Organic salt
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 284 - 287 °C | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ffx-7900000000-53446c0cac1ef1635285 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f6x-0900000000-0f08458ca2320e65e267 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f6x-0900000000-9d120e91bcc57d0c5524 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00m0-5900000000-1cded7c9b4492a2d4a0d | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f79-0900000000-be0ee749e164f60b0914 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-3900000000-c52c686f4d064b27b225 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gcc-9400000000-2007c980a67a00210879 | 2017-09-01 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB40265 |
|---|
| PubChem Compound ID | 23691036 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 19273 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Link |
|---|
| General References | - Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|