| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-11 05:21:46 UTC |
|---|
| Update Date | 2014-12-24 20:26:59 UTC |
|---|
| Accession Number | T3D4907 |
|---|
| Identification |
|---|
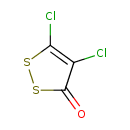
| Common Name | 4,5-Dichloro-3H-1,3-dithiol-2-one |
|---|
| Class | Small Molecule |
|---|
| Description | 4,5-Dichloro-3H-1,3-dithiol-2-one is a fda approved slimicide for use in food-contact paper and paperboard. |
|---|
| Compound Type | - Food Additive
- Food Toxin
- Metabolite
- Organic Compound
- Organochloride
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 4,5-Dichloro-1,2-dithia-4-cyclopenten-3-one | | 4,5-Dichloro-1,2-dithiacyclopentenone | | 4,5-Dichloro-1,2-dithiol-2-one | | 4,5-Dichloro-1,2-dithiol-3-one | | 4,5-Dichloro-3-oxo-1,2-dithiole | | 4,5-Dichloro-3H-1,2-Dithiol-3-one | | 4,5-Dichloro-3H-1,2-Dithiole-3-one | | Dichloro-1,2-Dithiol-3-one | | Dichlorodithiolone |
|
|---|
| Chemical Formula | C3Cl2OS2 |
|---|
| Average Molecular Mass | 187.068 g/mol |
|---|
| Monoisotopic Mass | 185.877 g/mol |
|---|
| CAS Registry Number | 1192-52-5 |
|---|
| IUPAC Name | dichloro-3H-1,2-dithiol-3-one |
|---|
| Traditional Name | dichloro-1,2-dithiol-3-one |
|---|
| SMILES | ClC1=C(Cl)C(=O)SS1 |
|---|
| InChI Identifier | InChI=1S/C3Cl2OS2/c4-1-2(5)7-8-3(1)6 |
|---|
| InChI Key | InChIKey=QGSRKGWCQSATCL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aryl chlorides. These are organic compounds containing the acyl chloride functional group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organohalogen compounds |
|---|
| Class | Aryl halides |
|---|
| Sub Class | Aryl chlorides |
|---|
| Direct Parent | Aryl chlorides |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,2-dithiole-3-one
- Aryl chloride
- Heteroaromatic compound
- Vinylogous halide
- Dithiole
- 1,2-dithiole
- Organoheterocyclic compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organochloride
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 61 °C | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-2900000000-982a924c03859d0e6885 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0900000000-f69d81a643f98cb33b77 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0900000000-f69d81a643f98cb33b77 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0900000000-f69d81a643f98cb33b77 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-aa9612aa1e7cce874b78 | 2016-08-04 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0900000000-aa9612aa1e7cce874b78 | 2016-08-04 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-0900000000-aa9612aa1e7cce874b78 | 2016-08-04 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0900000000-8765fbba636c0507f944 | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0900000000-8765fbba636c0507f944 | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dr-7900000000-ea182e329020e6a2639e | 2021-09-25 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB33151 |
|---|
| PubChem Compound ID | 14503 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 13847 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|