| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-11 05:22:21 UTC |
|---|
| Update Date | 2014-12-24 20:26:59 UTC |
|---|
| Accession Number | T3D4916 |
|---|
| Identification |
|---|
| Common Name | Fast green FCF |
|---|
| Class | Small Molecule |
|---|
| Description | FDA permitted food colourant used in beverages, desserts, candies, baking goods, dairy products and maraschino cherries Fast Green FCF, also called Food green 3, FD&C Green No. 3, Green 1724, Solid Green FCF, and C.I. 42053, is a sea green triarylmethane food dye. Its E number is E143. |
|---|
| Compound Type | - Amine
- Dye
- Ester
- Food Additive
- Food Toxin
- Household Toxin
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
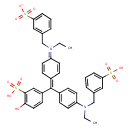
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Aizen Food Green No. 3 | | C.I. 42053 | | C.I. Food green 3 | | C.I. Food Green 3, disodium salt | | FD & C green no. 3 | | FD and C Green No. 3 | | FD&C Green No. 3 | | Food Green 3 | | Food Green No. 3 | | N-Ethyl-N-[4-[[4-[ethyl[(3-sulfophenyl)methyl]amino]phenyl](4-hydroxy-2-sulfophenyl)methylene]-2,5-cyclohexadien-1-ylidene]-3-sulfobenzenemethanaminium hydroxide inner salt, 9CI | | Solid green FCF | | Zelen potravinarska 3 | | Zelen stala FCF |
|
|---|
| Chemical Formula | C37H36N2O10S3 |
|---|
| Average Molecular Mass | 764.884 g/mol |
|---|
| Monoisotopic Mass | 764.153 g/mol |
|---|
| CAS Registry Number | 2353-45-9 |
|---|
| IUPAC Name | 3-{[ethyl({4-[(4-{ethyl[(3-sulfophenyl)methyl]amino}phenyl)(4-hydroxy-3-sulfophenyl)methylidene]cyclohexa-2,5-dien-1-ylidene})azaniumyl]methyl}benzene-1-sulfonate |
|---|
| Traditional Name | 3-{[ethyl({4-[(4-{ethyl[(3-sulfophenyl)methyl]amino}phenyl)(4-hydroxy-3-sulfophenyl)methylidene]cyclohexa-2,5-dien-1-ylidene})ammonio]methyl}benzenesulfonate |
|---|
| SMILES | CCN(CC1=CC(=CC=C1)S(O)(=O)=O)C1=CC=C(C=C1)C(C1=CC(C(=O)C=C1)=S(O)(O)=O)=C1C=CC(C=C1)=[N+](CC)CC1=CC(=CC=C1)S([O-])(=O)=O |
|---|
| InChI Identifier | InChI=1S/C37H36N2O10S3/c1-3-38(24-26-7-5-9-33(21-26)50(41,42)43)31-16-11-28(12-17-31)37(30-15-20-35(40)36(23-30)52(47,48)49)29-13-18-32(19-14-29)39(4-2)25-27-8-6-10-34(22-27)51(44,45)46/h5-23H,3-4,24-25H2,1-2H3,(H3,41,42,43,44,45,46,47,48,49) |
|---|
| InChI Key | InChIKey=MNEJSIQJOSRAAA-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylbenzamines. These are aromatic compounds consisting of a benzyl group that is N-linked to a benzamine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenylmethylamines |
|---|
| Direct Parent | Phenylbenzamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylbenzamine
- Diphenylmethane
- Benzenesulfonate
- Arylsulfonic acid or derivatives
- 1-sulfo,2-unsubstituted aromatic compound
- Benzenesulfonyl group
- Tertiary aliphatic/aromatic amine
- Dialkylarylamine
- Aniline or substituted anilines
- Benzylamine
- Aralkylamine
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Azomethine
- Secondary ketimine
- Sulfonyl
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Organosulfonic acid
- Tertiary amine
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Amine
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 290 °C | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-007p-4400048900-2a0b377a244f42604375 | 2017-11-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-10-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-10-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-10-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-10-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-10-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-10-18 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000001900-6cce1074692231b16621 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0000004900-1d0c3efc282014e7b154 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mp-1010092000-aadcf847abea612216e2 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000000900-eb3a62ffaa0a9eb7d316 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-1000000900-eab8a71b39d9d909735d | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9000003200-4552ca36d4f571e8d653 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000000900-4105248b4b31315b270c | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0000002900-1444299cffab2015d01c | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pc0-2100019400-3be92d8a1e2c1701d94f | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014j-0000012900-faaf95e7d7947d437cf0 | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0536-2000029200-e7b442b13214b42204f7 | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a59-0401039200-6ae09df02c7aeb43f530 | 2021-09-25 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 3, not classifiable as to its carcinogenicity to humans. (2) |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB38220 |
|---|
| PubChem Compound ID | 3400691 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 2645075 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Fast_green_FCF |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D4916.pdf |
|---|
| General References | - Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|