| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-17 20:01:58 UTC |
|---|
| Update Date | 2014-12-24 20:27:00 UTC |
|---|
| Accession Number | T3D4946 |
|---|
| Identification |
|---|
| Common Name | 1-Methyl-2-pyrrolidone |
|---|
| Class | Small Molecule |
|---|
| Description | 1-Methyl-2-pyrrolidone, or N-Methyl-2-pyrrolidone (NMP), is a chemical compound with 5-membered lactam structure. It is a colorless to slightly yellow liquid miscible with water. It is used in petrochemical processing, and as a solvent for surface treatment of textiles, resins and metal coated plastics or as a paint stripper. In the pharmaceutical industry, N-methyl-2-pyrrolidone is used in the formulation for drugs by both oral and transdermal delivery routes. NMP has been identified as a reproductive toxicant, first by California in 2001[3] and then by the European Commission in 2003. In the face of increasing regulation, some manufacturers are considering alternative solvents for some applications, especially where worker exposure is difficult to control, such as in paint stripping, graffiti removal, and agriculture. (Wikipedia) |
|---|
| Compound Type | - Amide
- Amine
- Household Toxin
- Industrial/Workplace Toxin
- Organic Compound
- Solvent
- Synthetic Compound
|
|---|
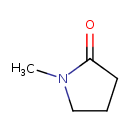
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1-Methyl-2-pyrrolidinone | | 1-Methylazacyclopentan-2-one | | N-Methyl-alpha-pyrrolidinone | | N-Methyl-alpha-pyrrolidone | | N-Methyl-gamma-butyrolactam | | NMP |
|
|---|
| Chemical Formula | C5H9NO |
|---|
| Average Molecular Mass | 99.131 g/mol |
|---|
| Monoisotopic Mass | 99.068 g/mol |
|---|
| CAS Registry Number | 872-50-4 |
|---|
| IUPAC Name | 1-methylpyrrolidin-2-one |
|---|
| Traditional Name | methylpyrrolidone |
|---|
| SMILES | CN1CCCC1=O |
|---|
| InChI Identifier | InChI=1S/C5H9NO/c1-6-4-2-3-5(6)7/h2-4H2,1H3 |
|---|
| InChI Key | InChIKey=SECXISVLQFMRJM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-alkylpyrrolidines. N-alkylpyrrolidines are compounds containing a pyrrolidine moiety that is substituted at the N1-position with an alkyl group. Pyrrolidine is a five-membered saturated aliphatic heterocycle with one nitrogen atom and four carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyrrolidines |
|---|
| Sub Class | N-alkylpyrrolidines |
|---|
| Direct Parent | N-alkylpyrrolidines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrrolidone
- 2-pyrrolidone
- N-alkylpyrrolidine
- Tertiary carboxylic acid amide
- Carboxamide group
- Lactam
- Carboxylic acid derivative
- Azacycle
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Microsome
- Microtubule
- Mitochondrion
- Plasma Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Quinolones | Not Available | Not Available | | Apoptosis | Not Available | map04210 | | Nicotine addiction | Not Available | map05033 | | Mismatch repair | Not Available | map03430 | | Cell cycle | Not Available | map04110 |
|
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Colorless to slightly yellow liquid. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | -24°C | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0007-9000000000-f6b5ec5cf179d5b2b4e3 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0005-9000000000-47ce7569060cdb946f8c | 2017-09-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0udi-0900000000-b144630f86d4b8e66518 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0udi-0900000000-374f2f08e213ac620908 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0zfr-8900000000-49455aab0a6c37691a02 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0a4i-9000000000-db81a1cd9771bdf5636c | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0a4i-9000000000-d604c3fdefd449d36b13 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0udi-0900000000-dabd1db3f0942df68f1f | 2017-09-14 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0900000000-ce64e8af2b11f9d10358 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-3900000000-994475260f0994c425c9 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0536-9000000000-84a641fdb9dbe809d098 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-9000000000-900ff8933c879631d09d | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9000000000-cd5028e268ba08ceadb2 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9000000000-7f6caecde6fc71b63720 | 2016-08-03 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0007-9000000000-6f78d4926f0506c5f52f | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 25.16 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | 1-Methyl-2-pyrrolidone is rapidly biotransformed by hydroxylation to 5-hydroxy- N -methyl-2-pyrrolidone, which is further oxidized to N -methylsuccinimide; this intermediate is further hydroxylated to 2-hydroxy- N - methylsuccinimide. These metabolites are all colourless. The excreted amounts of NMP metabolites in the urine after inhalation or oral intake represented about 100% and 65% of the administered doses, respectively. |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | 1-Methyl-2-pyrrolidone is used in petrochemical processing, and as a solvent for surface treatment of textiles, resins and metal coated plastics or as a paint stripper. In the pharmaceutical industry, N-methyl-2-pyrrolidone is used in the formulation for drugs by both oral and transdermal delivery routes. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Rapid, irregular respiration, shortnessof breath, decreased pain reflex, and slight bloody nasalsecretion. |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| ChEMBL ID | CHEMBL12543 |
|---|
| ChemSpider ID | 12814 |
|---|
| KEGG ID | 13387 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D4946.pdf |
|---|
| General References | Not Available |
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|