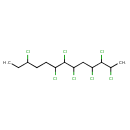| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-17 20:23:40 UTC |
|---|
| Update Date | 2014-12-24 20:27:00 UTC |
|---|
| Accession Number | T3D4954 |
|---|
| Identification |
|---|
| Common Name | 2,3,4,6,7,8,11-Heptachlorotridecane |
|---|
| Class | Small Molecule |
|---|
| Description | 2,3,4,6,7,8,11-Heptachlorotridecane is a short chain chlorinated paraffin (SCCP). Chlorinated paraffins are synthetic compounds used primarily as coolants and lubricants in metal forming and cutting, in addition to being used as plasticizers and flame retardants in rubber, paints, adhesives, sealants and plastics. SCCPs (C10РC13 chloroalkanes) were included in 2008 in the European Chemicals Agency (ECHA) list of Substances of Very High Concern (SVHC) due their being a PBT (persistent, bioaccumulative and toxic) and vPvB (very persistent and very bioaccumulative) class of compounds. Substances in the list of SVHCs are those for which ECHA is considering imposing a requirement for authorization for some or all uses. Acute toxicity of SCCPs (C10-13) is very low. SCCPs may cause skin and eye irritation upon repeated application, but do not appear to induce skin sensitization (1). Chlorinated paraffins (average chain length C12; approx. 60% chlorine by weight) are listed on the International Agency for Research on Cancer's (IARC) Carcinogen List as 'Possible Carcinogens.' On the U.S. National Toxicology Program (NTP) Carcinogen List they are listed as 'Reasonably Anticipated to Be a Carcinogen.' There are no data on fertility or developmental effects for humans. No changes in reproductive organs were observed in a 13 week study with rats and mice dosed with 5000 and 2000 mg/kg/day of an SCCP. In addition, developmental effects were observed in rats at 2000 mg/kg/day but not at lower doses (1). (2) |
|---|
| Compound Type | - Coolant
- Industrial/Workplace Toxin
- Lachrymator
- Organic Compound
- Organochloride
- Plastic
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C13H21Cl7 |
|---|
| Average Molecular Mass | 425.477 g/mol |
|---|
| Monoisotopic Mass | 421.946 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 2,3,4,6,7,8,11-heptachlorotridecane |
|---|
| Traditional Name | 2,3,4,6,7,8,11-heptachlorotridecane |
|---|
| SMILES | CCC(Cl)CCC(Cl)C(Cl)C(Cl)CC(Cl)C(Cl)C(C)Cl |
|---|
| InChI Identifier | InChI=1/C13H21Cl7/c1-3-8(15)4-5-9(16)13(20)11(18)6-10(17)12(19)7(2)14/h7-13H,3-6H2,1-2H3 |
|---|
| InChI Key | InChIKey=HVAZUEGTYITCDZ-UHFFFAOYNA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as organochlorides. Organochlorides are compounds containing a chemical bond between a carbon atom and a chlorine atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organohalogen compounds |
|---|
| Class | Organochlorides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Organochlorides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydrocarbon derivative
- Organochloride
- Alkyl halide
- Alkyl chloride
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0216900000-3bcbd21aa88ce683222c | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-1216900000-f50c79cce570288056bd | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0r90-6920000000-c0999b804b59b5985855 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0964400000-21bc4f52070e4c280f48 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001j-0009100000-b82238cdca8d35f5d7c1 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05cs-5898000000-b00221f92b1938bac7fb | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 2B, possibly carcinogenic to humans. (3) |
|---|
| Uses/Sources | Chlorinated paraffins are used primarily as coolants and lubricants in metal forming and cutting, in addition to being used as plasticizers and flame retardants in rubber, paints, adhesives, sealants and plastics. (2) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | Not Available |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - United Nations Environment Programme. (UNEP). Stockholm Convention on Persistent Organic Pollutants (POPs). Persistent Organic Pollutants Review Committee. Revised Draft Risk Profile: Short-Chained Chlorinated Paraffins. 9 July 2009. [Link]
- Toxipedia - Chlorinated Paraffins [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|