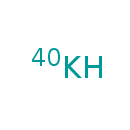| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-03-06 18:58:18 UTC |
|---|
| Update Date | 2014-12-24 20:21:21 UTC |
|---|
| Accession Number | T3D0217 |
|---|
| Identification |
|---|
| Common Name | Potassium-40 |
|---|
| Class | Small Molecule |
|---|
| Description | Potassium-40 is a naturally occurring radioactive isotope of potassium. Potassium is the chemical element with the symbol K and atomic number 19. Potassium is widely distributed in nature and is present in all plant and animal tissues. Potassium-40 comprises about 0.012% of naturally occurring potassium. It is the predominant radioactive component in human tissues and in most food. Potassium-40 has a half-life of 1.3 billion years and emits beta and gamma radiation. (1, 3) |
|---|
| Compound Type | - Food Toxin
- Inorganic Compound
- Metal
- Natural Compound
- Pollutant
- Radioactive
- Radioactive Isotope
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (40)K | | 40K | | Potassium 40 | | Potassium, isotope of mass 40 |
|
|---|
| Chemical Formula | K |
|---|
| Average Molecular Mass | 39.964 g/mol |
|---|
| Monoisotopic Mass | 39.964 g/mol |
|---|
| CAS Registry Number | 13966-00-2 |
|---|
| IUPAC Name | (⁴⁰K)potassium |
|---|
| Traditional Name | (⁴⁰K)potassium |
|---|
| SMILES | [40K] |
|---|
| InChI Identifier | InChI=1S/K/i1+1 |
|---|
| InChI Key | InChIKey=ZLMJMSJWJFRBEC-OUBTZVSYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Classification | Not classified |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | Silvery-white metal. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | Not Available |
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (1) ; inhalation (1) |
|---|
| Mechanism of Toxicity | The ionizing radiation produced by potassium causes cellular damage that includes DNA breakage, accurate or inaccurate repair, apoptosis, gene mutations, chromosomal change, and genetic instability. This leads to loss of normal cell and tissue homeostasis, and development of malignancy. Ionizing radiation that does not directly damage DNA can produce reactive oxygen intermediates that directly affect the stability of p53, an important enzyme in cell-cycle regulation, and produce oxidative damage to individual bases in DNA and point mutations by mispairing during DNA replication. (2) |
|---|
| Metabolism | Potassium-40 behaves in the body in the same manner as other potassium isotopes. Potassium is almost completely absorbed upon ingestion, moving quickly from the gastrointestinal tract to the bloodstream. The potassium-40 that enters the bloodstream after ingestion or inhalation is quickly distributed to all organs and tissues. Potassium-40 is eliminated from the body with a biological half-life of 30 days. The potassium content of the body is under strict homeostatic control (in which the amount retained is actively regulated by the body to achieve the normal range required for system functions), and it is not influenced by variations in environmental levels. Hence, the potassium-40 content in the body is constant, with an adult male having about 0.1 microcurie or 100,000 pCi. Each year this isotope delivers doses of about 18 millirem (mrem) to soft tissues of the body and 14 mrem to bone. Potassium cations are important in neuron function, influencing osmotic balance between cells and the interstitial fluid, allowing muscle contraction and the sending of all nerve impulses through action potentials, and maintaining fluid and electrolyte balance in the body. (1, 3) |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Internalized radionuclides that emit β particles are carcinogenic to humans (Group 1) (5). Potassium-40 undergoes beta decay. |
|---|
| Uses/Sources | There are no specific commercial or medical uses associated with the radioactive properties of potassium-40. (1) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Potassium-40 presents external as well as internal health hazard. The strong gamma radiation makes external exposure to this isotope a concern. While in the body, potassium-40 poses a health hazard from both the beta particles and gamma rays. The health hazard of potassium-40 is associated with cell damage caused by the ionizing radiation that results from radioactive decay, with the general potential for subsequent cancer induction. (1) |
|---|
| Symptoms | Exposure to high doses of ionizing radiation results in acute radiation syndrome, which can cause skin burns, hair loss, nausea, vomiting, dizziness, disorientation, low blood pressure, headache, fatigue, weakness, fever, birth defects, illness, infection, and death. (2, 4) |
|---|
| Treatment | Treatment reversing the effects of irradiation is currently not possible. Anaesthetics and antiemetics are administered to counter the symptoms of exposure, as well as antibiotics for countering secondary infections due to the resulting immune system deficiency. (4) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 6328542 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 4886606 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Potassium-40 |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - Argonne National Laboratory, EVS (2005). Human Health Fact Sheet, Potassium-40. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (1999). Toxicological profile for ionizing radiation. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Potassium. Last Updated 23 August 2009. [Link]
- Wikipedia. Radiation poisoning. Last Updated 22 August 2009. [Link]
- International Agency for Research on Cancer. 2012. Radiation: A Review of Human Carcinogens. IARC monograph, volume 100D. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|